Abstract
Содержание
- Introduction
- 1. The relevance, purpose and tasks of the research and planned results
- 2. General characteristic glauconite
- 3.Fiziko – chemical basis of the adsorption process
- 4. The experimental procedure
- Conclusion
- References
Introduction
Contaminate natural water reservoirs as a result of human activities today is to one of the most urgent problems whose solution requires a cohesive efforts by the world scientific community. The current pace population growth in the world certainly will entail further increase in of industrial production, energy industry, increase area planted in agriculture development of urban infrastructure. The development of these industries requires intensive exploitation water resources is inevitable due to increased pressure on water bodies due to effluent discharge. Careless attitude towards to this problem provokes a disastrous consequences that acquire global scale [1].
Water resources of the Donetsk region formed by the transit flow of surface water, mainly on the Seversky Donets River through the Kharkiv region, local runoff, sewage, mine and quarry water, and groundwater storage (1,067 thousand cubic meters. Meters per day) . Fresh water is used the metallurgy, coal mining, energy, utilities and agriculture. The main pollutants of water bodies are the coal–mining and metallurgical industries. Every year, they dump about 500 million cubic meters. m mine water contaminated with mineral salts, suspended solids and bacterial contaminants. In the small rivers of the Donetsk region annually receives about 1.5 million tons of salt, which led to the drying up of rivers in recent years by one meter [2].
The category of major companies – pollutants steel industry should include the following: Metallurgical Combine. Ilyich Iron & Steel Works and in Mariupol metallurgical and coke–chemical plants in Yenakievo Alchevsk Iron and Steel Works, Alchevsk Stakhanovsky and coke plants. It should also be noted that because of the frequent accidents at facilities sanitation, their health and well being of remains unsatisfactory [3].
Thus, the use for long time classic of water purification techniques have led to increased pollution hydrospherewhich requires new strategies and wastewater treatment technologies [4].
1. The relevance, purpose and tasks of the research and planned results
In waste water treatment sorption methods use as an adsorbent activated carbon. It is advisable and rationally replace it by natural sorbents, which on one hand is inexpensive and accessible materials, and with another – enable reach a high degree purification. Relevant is the use of natural sorbent – glauconite [4].
The aim of the work is the experimental study of the sorption properties of qualitative and quantitative characteristics of the of natural mineral – glauconite Amvrosievskaya birth.
In order to achieve goal the following objectives:
1 – to conduct technical analysis of of glauconite;
2 – to investigate experimentally sorption activity of glauconite according to standard procedures in respect of methyl orange, of methyl red and of methylene blue;
3 – determine sorptive properties glauconite relative to heavy ones metals and inorganic ions;
4 – to investigate the sorption properties of of glauconite based on organic substances.
2. General characteristic glauconite
Glauconite refers to a group of aluminosilicates. Aluminosilicate – group of natural and synthetic of silicates, complex anions of which containing silicon and aluminum. Cations act as Na+, K+, Mg2+, Ca2+, Ba2+ and sometimes and Li+. Natural aluminum silicates are the most common minerals, which account for 50 % of the mass of the crust. These include feldspar (albite, orthoclase, anorthite), clay minerals and mica. Aluminum silicates do not dissolve in water. Natural aluminosilicates contain no hydroxyl group and the crystal water is refractory, thermally stable compounds. Glauconite is a natural natural mineral contained in sedimentary rocks. Modern environmental technology found that glauconite has a number of useful generic properties that can be used in different spheres of life. The uniqueness of this mineral is its high ion exchange, buffering and sorption properties.
Glauconite exists in the the form of small, rounded green beans. Is common throughout the geological systems – in the sands, sandstones, clays, marls and limestones, gives color to greenish in color. Formation of and glauconite is is currently on the seabed with the participation of of small organisms. Hardness on a mineralogical scale of 2 – 3, density of 2.2 – 2.8 g/cm3. Glauconite is promising minerals versatile use. Found four or forms of finding it in the Paleogenic sediments typomorphic five and three genetic variations (allogenic dalneprinosnoy, allogenic and authigenic relic). In authigenic glauconite is defined more than 50 chemical elements, the ratio of which reflect the paleogeograficheskie conditions glaukonizatsii. Established that when filtering of polluted water through it practically completely are removed the iron compound and ammonia, one order of magnitude decrease in the water content oil products in the 25 – 50–fold content is reduced of radioactive isotopes cesium–137 and strontium–90. Glauconitenonflammable, non–toxic, non–volatile, does not dissolve in water, of dilute acids and alkalis. Area of chemical resistance pH 1 – 10. Storage life of natural sorbent glauconite is not limited. Glauconite has a high adsorption and ion exchange properties. The mineral has the property selective absorption of of cations and long lived radioisotopes [5].
The ability of of glauconite adsorb heavy metals are from the solutions of (vi% of the initial content) Pb – 99, Hg – 64, Co – 97, Cu – 96, Cd – 96, Mn – 95, Cr – 92, Ni – 90 Zn – 90, Fe – 99. Sorbent has a good operational indicators and, as a consequence does not require additional measures for wash the column and its overload. Since the glauconite has a large active surface, it is selectively expressed adsorbs ammonia, hydrogen sulfide, methane, carbon dioxide, hydrocarbons, phenols, exo–and endotoxins, heavy metals, radionuclides, some microorganisms. Glauconite is able to absorb and excrete some Cations, being thus an additional source of mineral elements. Metals having a high atomic mass desorbed is significantly worse than the lungs. Also, glauconite can rid the body of heavy metal salts [6].
One use of glauconite is application of it on solid surface and soils exposed long–term pollution with petroleum products with heavy metals. Glauconite advantage is that after cleaning contaminated sites, a certain amount of sorbent in the concentration harmful substances reach the level of MPC, ie class waste hazard reduced down to a safe. The treated area must not sanitize. Thus peeled sorbent the soil remains in place and does not move to the landfill of hazardous waste, eliminates the extra cost of its disposal. Thus, glauconite expedient to use as an adsorbent for the extract of natural and waste waters of various pollutants [7].
3.Fiziko – chemical basis of the adsorption process
Sorption methods of are highly effective for the extract of wastewater of dissolved substances with subsequent recycling and use of treated wastewater recycled water supply systems in industrial plants. Basic information about the sorption material properties and the nature adsorption of of certain substances can be prepared from adsorption isotherms of characterizing dependence the concentration the sorption capacity (or pressure), the component is adsorbed at a constant temperature. Brunauer, Emmett and Teller [8]identified five major types of sorption isotherms, the so–called BET isotherms. Convex portions isotherms I, II and IV types indicate the presence of micropores in the sorbent, but, moreover, sorbents II and IV also have macroporosity. Isotherms of types III and V are less common and represent a strong intermolecular interactions in matter sorbate.
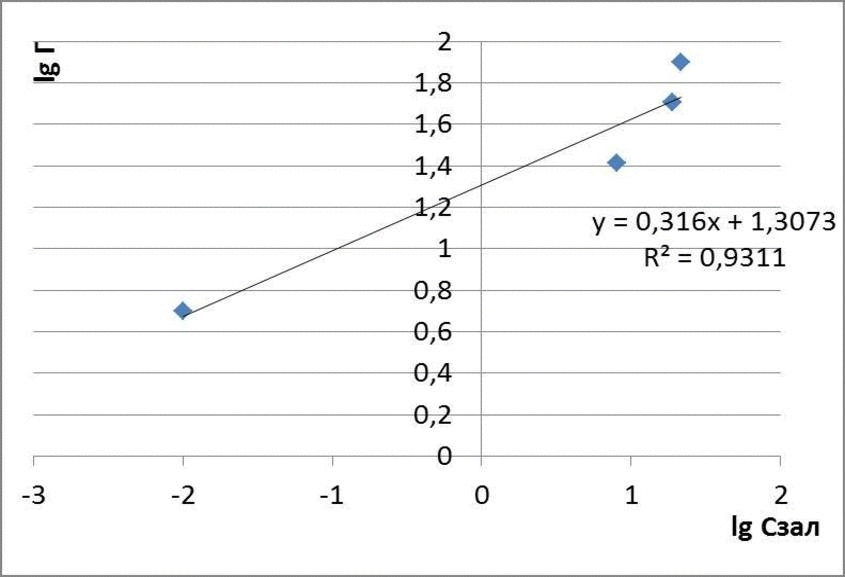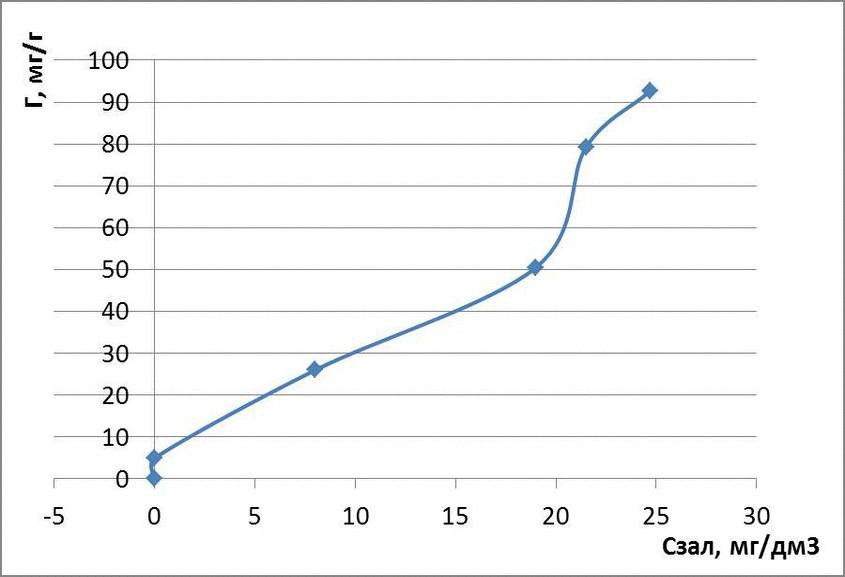
In the intermediate equilibrium concentrations (in small areas of change in the concentration of the adsorbate), the dependence of adsorption the concentration can often be described by the Freundlich equation, which is based on the assumption that the adsorption isotherm is a parabola. In logarifmovannomu form of Freundlich equation is straightforward.
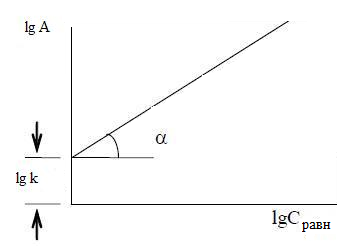
Figure 3.1 – Scheme of determination of the constants in the equation Frendliha
А = к · С1/n ,
where к и 1/n – constants.
The intercept on the ordinate axis is equal to lg, tg? = 1/n. In practice, there are other, more complex types of isotherms corresponding to the individual properties of the adsorbent and the adsorptive [9]. Thus, a complex heterogeneous adsorption process whose efficiency depends on many factors.
4. The experimental procedure
To determine the sorption capacity of glauconite series of experiments were conducted according to standard procedures [10]. According to [10] determined the sorption activity of glauconite fraction (1 - 3) mm with respect to the methyl red, methyl orange and methylene blue. Glauconite sample weight of 0.1 g were placed in a 100 ml flask and 25 ml of dye solution concentration of 1500 mg/dm3. Glauconite then separated from the solution and the absorbance found to methyl orange with a wavelength of 400 nm, methyl red – 490 nm for methylene blue – 590 nm. The residual dye concentration was determined by the colorimetric method using a calibration graph. Adsorptive activity (X) in milligrams per 1 gram of product was calculated using the formula:
Х = (С1 – С2 · К) · 0,025 / m
where C1 – concentration of the dye stock solution, mg/dm3;
C2 – the concentration of the dye solution after contact with activated carbon, mg/dm3;
K – coefficient of dilution;
m – mass of activated carbon sample, g;
0,025 – the amount of the dye solution, participating in the study, dm3.
The data obtained adsorption isotherms in logarithmic coordinates natural and presented in figures 4.1 – 4.3
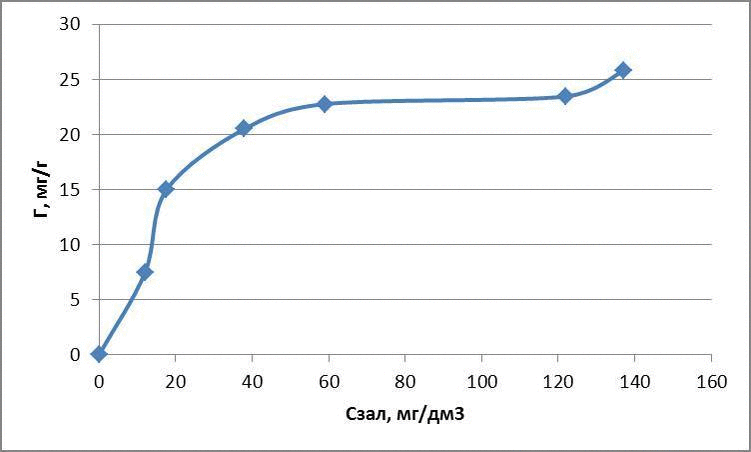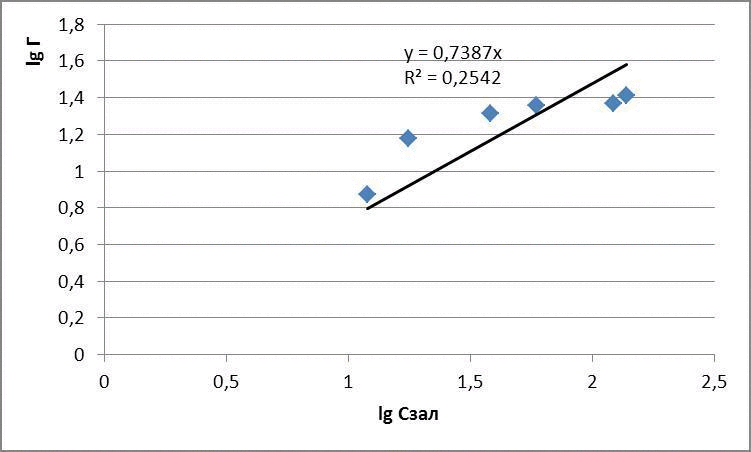
figures 4.1 – The adsorption isotherms of methylene blue
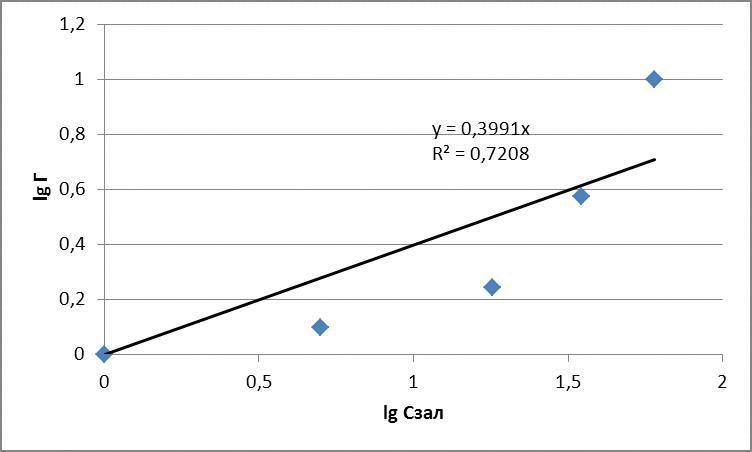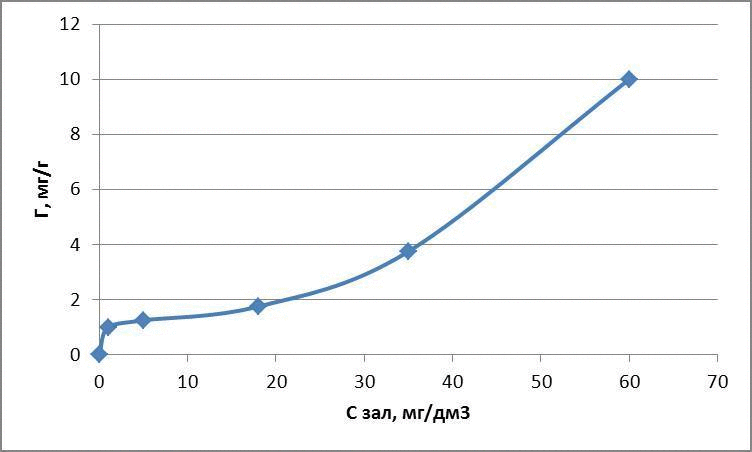
figures 4.2 – The adsorption isotherms of methyl red
figures 4.3 – The adsorption isotherms of methyl orange
Thus, as a result of the experiments were obtained Freundlich equation:
for methyl orange: Г = 0,11 · С1,05
for methyl red: Г = 0,096 · С0,44
for methylene blue: Г = 1,31 · С0,7
Conclusion
The results of the work performed to the following conclusions:
1 – considered a general description of glauconite, are the main physical and chemical properties of the mineral. Proven versatility in a variety of industries.
2 – The physicochemical basis of the adsorption process, the basic equations, from which we find the adsorption constants, factors affecting the adsorption process.
3 – to experimentally examine the sorption properties of glauconite in relation to dyes. Glauconite obtained sorption activity compared with activated carbon of 60 to 80 %. Submitted Freundlich equation indicate a high intermolecular relationships between the adsorbent and adsorbate.
4 – proved promising and efficient use of natural sorbents, in particular glauconite for wastewater treatment proved its advantages over the other, namely, availability, cost, the possibility of multiple use and without regeneration.
In the future we plan to study the sorption activity of glauconite with respect to heavy metals and organic pollutants.
References
- Івченко, В.Д. Очищення стічних вод від іонів амонію та феруму глинистими мінералами Сумської області: Автореф. канд. техн. наук: 21.06.01 / Сумський національний аграрний університет – С., 2012. – 35 с.
- Зубков Р.M., Матлак Е.С. Екологічна обстановка в донецькій області / / Одеський гідрометеорологічний інститут. Матеріали III Всеукраїнської наукової студентської конференції "Екологічні проблеми регіонів" (м. Одеса, 25–26 квітня 2001 р.) – с. 30–32.
- Зубков Р.M., Редько А. Л. Екологічні проблеми донецько–макіївської промислово–міської агломерації // Вісник Донбаської державної академії будівництва та архітектури. Збірник наукових праць. Випуск 99–4 (18). Матеріали XXV студентської науково–технічної конференції студентів (27–28 квітня 1999 р.) – с. 78.
- Литвин, Т.С. Сорбційна активність глауконіту Амвросіївського родовища / О.А. Трошина, І.В. Качур, Т.С. Литвин // Матерiали ХХIIІ Всеукраїнської наукової конференцiї аспiрантiв і студентів. – Т.1 – Донецьк: ДонНТУ, 2013. – С. 80 – 81
- Запольський, А.К. Водопостачання, водовідведення та якість води: Підручник / А. К. Запольський. – К.: Вища школа, 2005. – 671 с.
- Таубаева Э.С. Сорбционные свойства глауконита / Э.С. Таубаева, У.Ж. Джусипбеков, С.М. Жунусов
- Фізико–хімічні основи технології очищення стічних вод / За редакцією А.К. Запольського. - Київ: Лібра, 2000. – 549 с.
- Смирнов, А.Д. Сорбционная очистка воды / А.Д. Смирнов. – Л.: Химия, 1982. – 168 с.
- Когановский, А.М. Адсорбция и ионный обмен в процессах водоподготовки и очистки сточных вод. / А.М. Когановский. – К.: Наукова думка, 1983. – 240 с.
- ГОСТ 4453 – 74. Уголь активный осветляющий древесный порошкообразный. Технические условия. М.: Государственный комитет стандартов, 1974. –11 с.
