Abstract
- Introduction
- 1. Theme urgency
- 2. Goal and tasks of the research
- 3. Сalorimetry
- Conclusion
- References
Introduction
Currently, natural gas is widely used as raw materials and fuel for different industries. Natural gas is the fuel of the best, second only to oil. This advantage is due to the high heat of the combustion gas is easily controlled feed into the furnace, the absence of ashes.
Gas – the most environmentally friendly fuel, so that it is used as an energy source more often than other types of fuel. But the amount due to the growth of the industry dramatically reduced, resulting in a problem of rational use of gas quality. It is known that the larger the specific heat of combustion, the lower the specific fuel consumption at the same efficiency index. Specific heat of combustion is measured in joules per kilogram, or calories per kilogram. For the experimental measurement of this quantity used calorimetry methods.
Calorimetry – the science that deals with the definition of change of energy by measuring the heat exchange with the environment. The instruments used for these measurements are called calorimeters. Standard tools for measuring the calorific value of solid and liquid combustible materials are oxygen bomb colorimeter.
1. Theme urgency
Due to the modern development of the applied areas of science and technology, there are actual work to the measurement technique. The most important place in the measurement technique is Calorimetry. With calorimeters determine the specific heat, thermal conductivity, calorific value, the amount of heat released when the state, enthalpy, entropy.
Calorimeters used in metallurgy, electronics, medicine, radiation dosimetry, physics, physical chemistry, the gasification. In recent years has developed a number of standard and exemplary systems for calorimetry. But, despite this, still remain some problems associated with the creation of technical calorimeters, which would satisfy the requirement of massive technical measurements for different application tasks calorimetric sensitivity, temperature threshold of sensitivity, speed, metrological characteristics.
All these parameters depend on the calorimeters of their thermal characteristics (nature of heat exchange with the environment), the design features of the calorimeters, the types of temperature sensors, the method of the calorimetric experiment. Therefore, to be specified classification of calorimeters, their characteristics, examination of the existing models, their comparison and subsequent development of the calorimeter with superior one features. Therefore, the work aimed at solving these problems is relevant and has great economic and industrial importance.
2. Goal and tasks of the research
The aim of this work is to develop a device that determines the calorific value of the gas to the modern base.
Main tasks of the research:
- Analysis of existing calorimeters, the research of their characteristics, comparative analysis instruments.
- Research and the search for solutions of various industrial and laboratory measurements in calorimetry.
- Assessment by increasing the speed, temperature range, sensitivity, threshold sensitivity, radiation transparency.
- Metrological research and evaluation.
- Introduction in research development and production practices.
Object of research : the process of measuring the calorific value of natural gas at the control points of the Russia–Ukraine.
Subject of research : an instrument for measuring the calorific value of the gas.
3. Сalorimetry
The main objective of the use of natural gas is its rational consumption, ie the reduction of specific consumption through the implementation of the economic process, in which most fully realized the positive properties of the gas.
use of gas fuel to avoid heat loss, determined by chemical and mechanical incomplete combustion. Reducing heat loss from leaving the combustion products is achieved by burning the gas at low coefficient of discharge air.
When the gas-fired units may also use a stepped combustion products. To ensure that the use of gas was the most efficient, it is necessary to determine its calorific value. This is why there are colorimeters.
calorimeter is a table-top device. Operation calorimeter is measuring the change in temperature of the calorimeter with a known energy equivalent in burning a fixed amount of the test fuel.
Specific heat of combustion is determined by the formula:
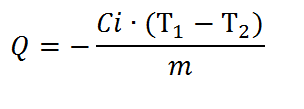
where С i – the energy equivalent of the calorimeter, T 1 – the theoretical value, T 2 – experimental value, m – mass of the fuel sample.
In calorimetry M-08 temperature change produced by metastatic thermometer and optical devices.
Calories (combustion heat) of a sample can be determined broadly as the amount of heat liberated units. sample weight during combustion in oxygen in a closed constant volume. In this reaction, the sample and oxygen initially have the same temperature, and the combustion products are cooled to a few degrees above the initial temperature. water vapor formed during combustion is condensed to a liquid state. A more precise definition should be indicative of the temperature at which the reaction begins and ends.
However, the change of heat of combustion at the initial temperature of the possible variations are so insignificant that such details are not required. In addition, the onset temperature and the end is not the same, differing in the amount of temperature rise in the calorimeter – but the effect of this difference is so small that it is neglected. Consequently, the term caloric (or calorific value) measured in the bomb calorimeter means of heat released by combustion of carbon and hydrogen to oxygen and produce carbon dioxide and water, including the heat liberated during the oxidation of other elements (such as sulfur), which may present in the sample.
calorific value defined in an oxygen bomb calorimeter measured during displacement, wherein the heat derived from the sample is compared with the heat obtained from the combustion of this amount of acid, benzene or other standardized material known calorific value. These measurements are obtained by submitting the sample in oxygen under pressure in a pressure vessel of metal, so-called "bombs". The energy produced by this combustion is absorbed in the calorimeter. Here we fix the temperature changes in the absorbing medium. Then the heat of combustion of the sample is calculated by multiplying the temperature rise in the calorimeter at a pre-defined or the energy equivalent of heat, some in previous tests with the standardized material. It should introduce corrections to bring these values to any heat transfer occurring in the calorimeter, and take into account any adverse reactions that are unique in the combustion bomb.
Any bomb calorimeter must consist of four main parts:
1. A bomb or a vessel in which burning is combustible charge.
2. The box or container for immersion in the bomb measured quantity of water with a temperature sensor and a control mechanism.
3. Insulating sheath to protect the box from the transient thermal loads during the combustion process.
4. The thermometer or other sensor for measuring temperature changes in the container.
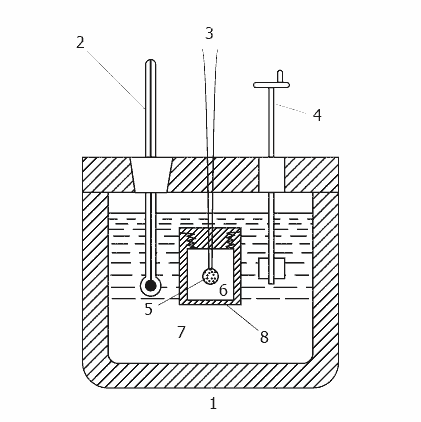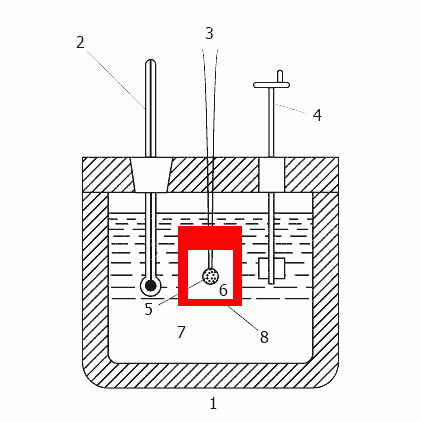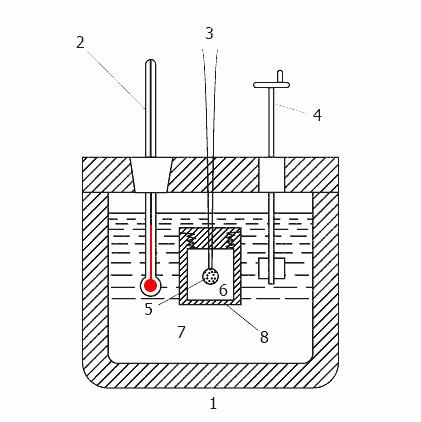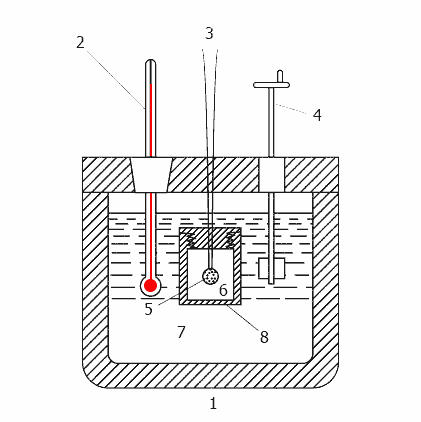
Animation - Operation calorimeter
(Animation: 11 frames, 13 cycles of repetition, 20 kilobytes)
1-insulated body, 2 - Thermometer, 3 - wire for ignition, 4 - agitator, 5 - sample 6 - oxygen, 7 - water,
8 - bomb
Вomb consists of a sturdy metal container with thick walls that can be opened to place the sample for extraction and combustion products for cleaning. Provision shall be made to fill the pipe bombs and oxygen under pressure for the release of the remaining gases after completion of the test. In addition, the bomb staffed electrodes for supplying current to the ignition wire fuse. Since during ignition internal pressure may reach up to 1500 psi, most oxygen bomb designed for pressures of at least 3000 psi.
Conclusion
In the oxygen bomb calorimeter is used several systems shells. The simplest of these are unregulated or envelopes with simple insulation. When using this type of membrane is assumed that the temperature of the shell, remains constant during the whole test without special adjustment of temperature. Given the simplicity, calorimeters with simple insulation is recommended as a low-cost devices for users who only occasionally carry out calorimetric test or for those users whose workload does not justify the cost of purchasing models with temperature control. Calorimeters with a simple wrapper does not require a constant connection. It can be set up and prepare for work in a few minutes if the calorimeter is not used, it can be easily dismantled and put in storage.
References
- Хеммингер В., Хене Г. Калориметрия. Теория и практика. М.: Химия. 1989. 176 с.
- Кальве Э., Прат А. Микрокалориметрия. М.: Изд-во ин. лит. 1963. 477 с.
- Washburn E.W. Standard states for bomb calorimetry. J. Res. Nat. Bur. Standards. 10. 1933. P. 525
- Попов М.М. Термохимия и калориметрия. 2-е изд. М.: 1954.
- Кирьянов К.В. Калориметрические методы исследования. Нижний Новгород, 2007, 76 с.
- Westrum E.F., Furukawa G.T., McCullough J.P. Adiabatic Low-temperature Calorimetry.Experimental Thermodynamics. V. I. Ed. McCullough J.P., Scott D.W. L.: Butterworths, 1968.
- Александров Ю.И., Осипова Т.Р., Юшкевич В.Ф. Исследование образцовых мер количества теплоты в калориметрии сжигания. Методы и средства калориметрии теплофизических измерений. Л., 1984. 250 с.
- Анатычук Л.И., Лусте О.Я. Микрокалориметрия. Львов: Вища школа. Изд-во при Львов. гос. ун-те. 1981. 160 с.
- Бернштейн В.А., Егоров В.М. Дифференциальная сканирующая калориметрия в физикохимии полимеров. Л.: Химия, 1990. 256 с.
- Вяхирев Р.А. Российская газовая энциклопедия, М: 2004, 527 с.
- Вайт П.А. Современная калориметрия, Л:1928, 340 с.
- Свитославский С.И. Микрокалориметрия, М:1946, 280 с.
- Сравнительный анализ и разработка дифференциальных, мостовых калориметров с термоэлектрическими и резистивными элементами [Электронный ресурс]. – Режим доступа: http://tekhnosfera.com/view/62460/a?#?page=20.
- История калориметрии . [Электронный ресурс]. – Режим доступа: http://www.kipstory.ru/pribori/color/.
