Abstract
Содержание
- Introduction
- 1. Actual themes
- 2. The purpose and goal of the research
- 3. Object of research
- 4. Experimental part
- 5. Discussion and results
- Conclusion
- References
- 5. Discussion and results
Introduction
It's need to understand the chemistry of the dissolution process, establish the relationship between the structure and the solubility of substances in a particular solvent for successful creation of a new conversion technologies of coal organic mass (COM) in liquid products.
When processing coal with low-boiling solvents output of extracts reaches up to 10 % for bituminous coals of low-level stage and 1 % for the middle-level stage of coal metamorphism. Numerous researches are aimed to increase their solubility, pursue and as a practical purpose – the rational use of the enormous hydrocarbon reserves, and the desire to explore the structure of fossil fuels. One method to increase the solubility is alkylation by alkyl halides in Lewis acids and coal slurry alkylating by hydroxyl tetraalkylammonium [1].
One of the new perspective methods for producing liquid products from solid fuels is thermochemolysis. At the same time, heat is used for thermochemolysis reactions, which leads to the catalytic cleavage of specific chemical bonds. The results of thermochemolysis are polar alkylated products with low molecular weight, which are easy to chromatographic analysis [2].
1. Actual themes
thermochemolysis is an improved form of analytical pyrolysis, which includes heating the sample in tetramethylammonium hydroxide. The method was developed by Bulgarian and French scientists applied to the peat and brown coal [3]. The main advantage of the method is that it allows for a larger number of liquid products compared to conventional dry
low-temperature pyrolysis of embodiments [4].
Efficiency of thermochimolisys is based on the methylation reactions in oxygen-containing group in combination with a thermal degradation temperature in range of 400–500 °C. This leads to intermolecular destruction in COM. Earlier thermochimolisys method was tested for coals researching [5].
2. The purpose and goal of the research
The purpose of research: a comparative study of structural-group composition of the standard carbonization of products, thermofiltration in the centrifugal field and polar fractions of liquid products thermochemolysis of Donetsk coals of different genetic types on restoration (TLG), identifying the relationship between the yield of liquid products of thermochemolysis reactions and structure of the original coal.
The research problem: destruction of intermolecular interactions between the oxygen-containing groups by methylation in conjunction with the thermal degradation in the temperature range 400–500 °C.
3. Object of research
Two pairs of Donetsk coals D-grade and J-grade classes type low reduced (LRC) and reduced (RC) were taken as an objects for research. Test coals were selected from nearby reservoirs mine Trudovskaya
, Gagarina
, the distance between them in the stratigraphic section was less than 100 m. This made possible to eliminate the influence of coal metamorphism to research results [6].
4. Experimental part
To solve the problem infrared Fourier transform spectroscopy and diffuse reflectance technique (DRIFT) were used. This makes possible to obtain the spectra of powders. This method of analysis is widely used in coal chemistry [7], etc. The big advantage of FT-IR method is the ability to use a computer for digital storage and processing of data. This allows spectra operations of expansion and contraction and their comparison or synthesis, factor and correlation analysis, displaying and printing curves programmed control experiment, baseline correction. Baseline method can partially eliminate the effects of deviations from the Beer-Lambert law, due to samples heterogeneity, the presence of larger particles compared to the wavelength, etc. [8]. The heat treatment was performed using different variants of the pyrolysis:
- under standard carbonization in Fisher retort;
- in HPI centrifuge;
- under thermochemolysis [5].
To use thermochemolysis method 1–2 grams of coal were placed in a ceramic boat, evenly distributed throughout the volume, few drops (0.1–0.2 ml) of tetramethylammonium as 25 % vol. solution in methanol were added and heated 12 hours to impregnation and evaporation of the solvent. Then the boat, together with the sample, moved to an oven preheated to 400 °C. Pyrolysis was carried out in flowing nitrogen (100 ml/min) during 1 hour. Liquid products thermochemolysis set aside in the refrigerator through a trap with chloroform, placed in an ice bath at −20 °C (Fig. 1).
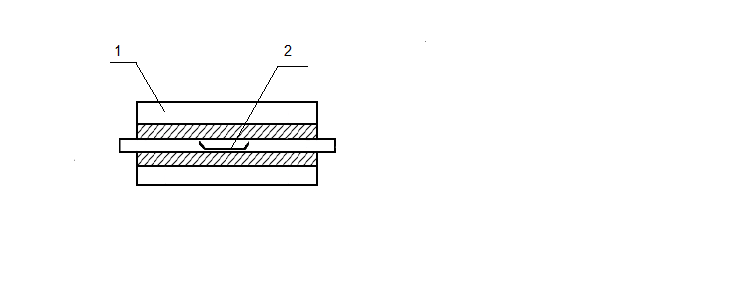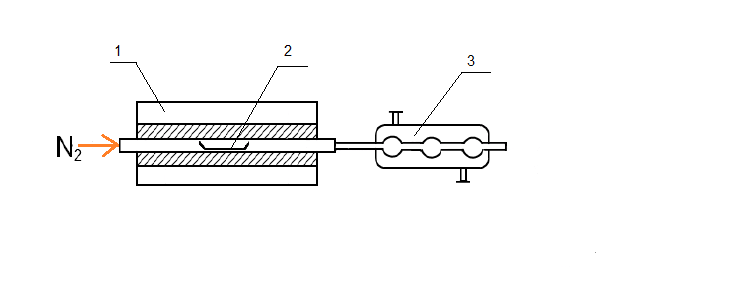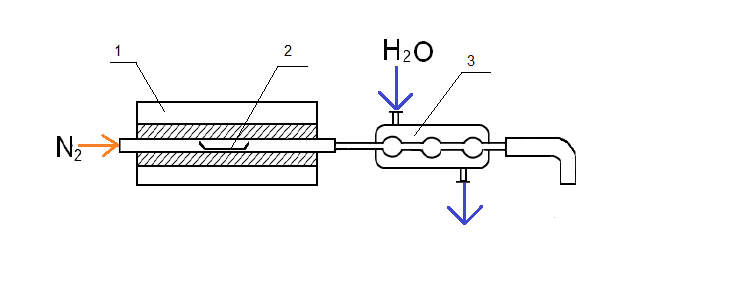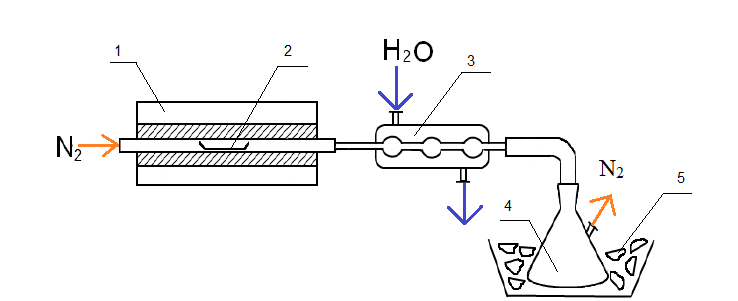
Figure 1 – Installation for thermochemolysis
(animation: 8 frames, 7 cycles of repetition, 42 kilobytes)
1 – furnace; 2 – boat with coal; 3 – refrigerator; 4 – trap with chloroform; 5 – ice bath.
Products separation was performed by column chromatography method. Silica was a stationary phase with a particle size of 0.2–0.5 mm, pre-activated at 200 °C for 2 hours. As eluents, mixtures of hexane and diethyl ether (Et2O) in different percentages were used. Next fractions were collected: 1st fraction – hexane; 2nd fraction – 10 % Et2O in hexane third fraction – 20 % Et2O in hexane; 4th fraction – 50 % Et2O in hexane; 5th fraction – polar
contains components, which are not soluble in these solvents [5].
First four fractions were researched by gas chromatography-mass spectrometry method. Fifth polar
fraction was researched using IR spectroscopy and Fourier Transform techniques diffuse reflectance (DRIFT) method, which takes a special place among the instrumental methods of coal and their thermal degradation processes research, as it allows you to receive high-quality spectra of these complex natural objects [5].
IR spectra of coal were recorded on Bruker
FTS-7 spectrometer, using DRIFT method. Baseline correction was performed using the computer program named Origin
. Baseline was made by local minimum points of the spectrum, which were recorded at a particular waves and are characteristic of all the IR spectra of coals. Structural analysis of coals and their DRIFT-spectra products was carried out by finding the characteristic absorption bands, referring them to the appropriate functional groups, semi-quantitative calculation of the intensities of the absorption bands and comparing them with the intensity of the band comparisons [9].
5. Discussion and results
Table 1.2 – Оf pyrolysis products and thermochemolysis coal, % daf
| Make | Type | Carbonization | Termofiltratsiya | thermochemolysis | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Char | Water | Resin | Gas | LNP | G | NR | Soluble products | SR | ||
| D-grade | LRC | 64.8 | 17.5 | 9.0 | 8.7 | – | – | – | 33.8 | 66.1 |
| J-grade | LRC | 84.4 | 2.3 | 5.3 | 8 | 22.4 | 19.9 | 57.7 | 24.9 | 75.1 |
| D-grade | RC | 62.1 | 10.7 | 14.3 | 12.9 | – | – | – | 50.6 | 49.4 |
| J-grade | RC | 71.8 | 1 | 4.7 | 22.5 | 38.7 | 21.3 | 40.0 | 34.3 | 65.7 |
Table 1.2 shows the results of yield products determination, derived from long-flame coals and coals of different TLG under carbonization (char, tar, water, gas); thermofiltration (LNP – liquid nonvolatile products, NR – nadsetochny residue G – gas) and thermochemolysis (soluble products and the solid residue). As can be seen from the table, the liquid yield long-flame coal carbonization is about 4 times lower comparing to the yield of products and thermofiltration thermochemolysis and for coals reduced type observed sevenfold excess. It is important to note that the yield of soluble products thermochemolysis close to the outlet thereof during thermofiltration [10]. Apparently, both methods implement maximum potential allocation of liquid products from COM.
Fractional composition method of thermochemolysis products researched in details. Table 1.3 shows the material balance of the process. As the table shows, the product yield affects the degree of metamorphism and TLG. The maximum amount of liquid products give coals D-grade of the reduced type. Then, according to dissolution, samples can be arranged in a row: D-gradeRC > J-gradeRC > D-gradeLRC > J-gradeLRC.
So, both characteristics of the coals have a tremendous impact on the yield of liquid thermochemolysis products. Intensity of the characteristic absorption bands (Ix) and relative values Ix/1580, Ix/2920 were determined by DRIFT-spectrum method, where strip of aromatic C=C bonds (1580–1600 cm−1) or Hal bonds (2920 cm−1) were used as a comparison. Absolute intensities of the absorption bands on the investigated products thermochemolysis spectra showed in table 1.4. Higher concentration of OH groups (band 3400 cm−1) draws attention to, which are primarily at methylation in long-flame coal thermochemolysis products [11].
Table 1.3 – Material balance of liquid products thermochemolysis
| Mine, reservoir | Make | Type | Yield of soluble products, % | Output fractions, % | Amount, % | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1th | 2th | 3th | 4th | 5th | Phenols | |||||
| Hexane | 10 % Et2O | 20 % Et2O | 50 % Et2O | Polar | ||||||
| Trudovskaya, l4 | D-grade | LRC | 33.84 | 2.8 | 3.1 | 2.8 | 3.0 | 36.2 | 52.1 | 100 |
| Gagarina, m3 | J-grade | LRC | 24.89 | 6.2 | 3.5 | 0.8 | 2.7 | 35.5 | 51.4 | 100 |
| Trudovskaya, k8 | D-grade | RC | 50.63 | 3.7 | 5.4 | 3.0 | 3.0 | 41.2 | 43.7 | 100 |
| Gagarina, m04 | J-grade | RC | 34.27 | 5.7 | 7.5 | 9.6 | 6.8 | 33.1 | 37.3 | 100 |
Table 1.4 – Absolute intensity of the absorption bands in the spectra of the polar fraction of liquid products thermochemolysis−1
| Sample | Absolute intensity of the bands | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 3400 | 3050 | 2950 | 2920 | 1740 | 1640 | 1580 | 1440 | 1380 | 1250 | 1150 | |
| J-gradeLRC | 0.019 | 0.035 | 0.067 | 0.056 | 0.011 | 0.008 | 0.009 | 0.030 | 0.011 | 0.019 | 0.008 | J-gradeRC | 0.015 | 0.023 | 0.058 | 0.055 | 0.140 | 0.110 | 0.057 | 0.066 | 0.039 | 0.050 | 0.036 | D-gradeLRC | 0.190 | 0.083 | 0.140 | 0.110 | 0.065 | 0.054 | 0.025 | 0.220 | 0.038 | 0.011 | 0.017 | D-gradeRC | 0.220 | 0.059 | 0.140 | 0.110 | 0.120 | 0.110 | 0.007 | 0.130 | 0.081 | 0.110 | 0.054 |
Table 1.5 shows results of relative intensity of the absorption bands on DRIFT-spectra of the 5th determination (not soluble fraction thermochemolysis products).
Table 1.5 – Relative intensity of the absorption bands on DRIFT-spectra of 5th fraction
| Sample | The relative intensity of the bands | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3400/ |
3050/ |
2950/ |
2920/ |
1740/ |
1640/ |
1440/ |
1380/ |
1250/ |
1150/ |
1740/ |
2950/ |
3050/ |
1250/ |
|
| J-gradeLRC | 1.95 | 3.61 | 6.91 | 5.77 | 1.13 | 0.82 | 3.09 | 1.13 | 1.95 | 0.82 | 0.19 | 1.19 | 0.63 | 0.34 | J-gradeRC | 0.26 | 0.40 | 1.02 | 0.96 | 2.45 | 1.93 | 1.15 | 0.68 | 0.87 | 0.64 | 2.55 | 1.05 | 0.42 | 0.91 | D-gradeLRC | 7.60 | 3.32 | 5.60 | 4.40 | 2.60 | 2.16 | 8.80 | 1.52 | 4.40 | 0.68 | 0.59 | 1.27 | 0.75 | 1.00 | D-gradeRC | 28.21 | 7.56 | 17.95 | 14.10 | 15.38 | 14.10 | 16.66 | 10.38 | 14.10 | 6.92 | 1.09 | 1.27 | 0.54 | 1.00 |
As seen from the table, the products obtained from the long-flame coal, in particular coal RC
were enriched oxygen-containing groups: hydroxyl (3400 cm−1), carbonyl (1640–1600, 1740 cm−1), ester (1250 cm−1). D-gradeRC coals have a relative concentration of OH, C=O, CHal groups 2–5 times higher comparing to with D-gradeLRC. Also active coal has a maximum content of aliphatic groups (band at 1440 cm−1, 1380 cm−1).
Comparation of different genetic types izometamorphic coals of on restoration shows that there are significant differences in their structural composition. Due to their low reduced all coals of J-grade, D-grade have a higher ratio Har/Hal. So, for sulfur coals such as RC
share of aliphatic fragments belonging to the macromolecules above (3050/2920 cm−1) is not dependent on the stage of metamorphism.
For most soluble coal D-gradeRC solid residue enriched by aromatic 3050/1580 cm−1 and bridging 1250/1580 cm−1, 1150/1580 cm−1 structures. The ratio of aliphatic bridging linkages is maximized.
Coal J-grade RC
, which has a maximum caking differences by minimum relative amount of short chains 2950/2920 cm−1 according to total number of CH3, CH2, CH groups and the minimum ratio of Har/Hal (3050/2920 cm−1) as well as a low content of ester bonds bridging and 1250 cm−1, 1150 cm−1. They, as is well known, impede the transition of coal to the plastic state.
Coal reduced products, recovered thermochemolysis give 1.5–2 times more compounds, that are soluble in hexane. According to GCMS in hexane eluent contains more alkanes (J-grade = 82.5 %, D-grade = 80.74 %).
The relative intensity of the bands I1740/I2920 – ratio of C=O groups to CH2, CH3, characterizes the strength of intermolecular interactions in the coals. This figure in recovered coal is higher than in weak-recovered. The maximum value of this parameter is observed for coal J-gradeRC, which has a high sintering ability and has a high yield of liquid thermofiltration products [10]. It was found, that this index correlates with the release of soluble products thermochemolysis. The magnitude of the correlation coefficient is 95 %. Thus, the determining factor for the transfer of coal in the soluble state is the relative content of C=O/C=C bonds in the COM (Fig. 2).
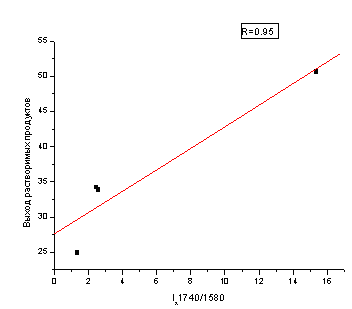
Figure 2 – Dependence of the soluble products of the intensity of the absorption bands of 1740/1580 cm−1
Conclusion
It is followed, that method of thermochemolysis of different genetic types for recovery Donbass coals allows to obtain a high yield of liquid products from. On the yield and composition of these products affects both brand coal and recovery-type coal, and high-sulfur coals form recovered significantly more liquid products, as part of which contains a smaller amount of phenol compared to izometamorphic weak-recovered coals.
Data makes conclusion that the main structural indicator, responsible for the formation of products thermochemolysis soluble in organic solvents , and is the ratio of oxygen-containing aromatic moieties in COM, which are measured by the ratio of the intensity of bands 1740/1580–1600 cm−1 DRIFT-spectra of fuels. Master's work is not yet complete. Final completion: December – 2014. Full text of the work and materials can be obtained from author or his manager after that date.
References
- Лазаров Л., Ангелова Г. Структура и реакции углей / София.: БАН, 1990. c. 231.
- Shsdkami F., Helleur R. Journal of analytical and applied pyrolysis thermochemolysis. – 2010. Vol. 89, pp. 3.
- Shadkami F., Helleur R. Recent Applications in Analytical Thermochemolysis / Journal of Analytical and Applied Pyrolysis – 2008. Vol. 39, pp. 32.
- Marinov S., Ivanov D., Yaneva N., Ambles A. Palaeoenvironment assessment of Pliocene Lom lignite (Bulgaria) from bitumen analysis and preparative off line thermochemolysis. – 2008. Vol. 39, pp. 1589–1605.
- Сафин В. А., Бутузова Л. Ф., Стефанова М., Коренкова И. Н. Термохимолиз разновосстоновленных углей Донбасса // Наукові праці Донецького національного технічного університету. Серія: Хімія і хімічна технологія / Донецк 2012, № 19, c. 123–125.
- Butuzova L., Safin V., Marinov S.,Yaneva N., Turchanina O., Butuzov G. The pathways for thermal decomposition of coals with high content of sulphur and oxygen / Geolines, Academy of Science of the Czech Republic, Vol. 22, pp. 15–19.
- Наливкина А. О., Маковский Р. В., Бутузова Л. Ф. Ик спектроскопическое изучение термообработаных шихт с разным содержанием серы. Наукові праці Донецького національного технічного університету. Серія: Хімія і хімічна технологія / Донецк, 2012 – № 19, c. 115–117.
- Бутузова Л. Ф., Турчанина О. Н., Скрипченко Г. Б. Влияние генетического типа по восстановленности на молекулярную и надмолекулярную организацию углей // Химия твердого топлива. – 2006 № 2, c. 20–29.
- Бутузова Л. Ф., Маковский Р. В., Маринов С., Семковский С. В. Поведение сернистых углей в шихте для коксования / ДонНТУ, 2011, c. 290.
- Маковский Р. В., Бутузова Л. Ф., Маринов С., Сафин В. А. Влияние cернистых компонентов на свойства угольных шихт при термической переработке // Наукові праці ДонНТУ. Серія: Хімія і хімічна технологія / Донецк, 2010 – № 14 (162), с. 97–103.
- Бутузова Л. Ф.,Маковский Р. В., Будинова Т., Бутузов Г. Н. Парамагнитные характеристики сернистых углей и шихт на их основе // Наукові праці Донецького національного технічного університету. Серія: Хімія і хімічна технологія / Донецк, 2010. – № 15 (163), с. 117–121.

