Abstract
Content
- Introduction
- 1. Physicochemical principles of process of paraffin formation and sedimentation
- 2. Physicochemical principles of process of hydrate formation
- Conclusions
- References
Introduction
Prospects of development of the gas industry in Russia are primarily concerned with the development of gas and gas condensate fields in the Far North. Transportation of gas from these fields is carried through pipelines located in the zone of permafrost. At the operating temperature pipelines impose stringent requirements to ensure their operational reliability and safety of the permafrost [1].
Condensates Achimov formations contain significant amounts of heavy hydrocarbon fractions. These heavy fractions contain substantial amounts of normal paraffin hydrocarbons. For this reason, at temperatures of 30 °C and lower the solid phase appears in condensates. When the temperature decreases the amount of solid phase increases. In the near future there will be a significant increase of the heavy paraffinic feedstock in total liquids production at the fields in the north of the Tyumen region, it will lead to the sediment of paraffins in piping systems. At the same time, the main condensate pipeline Urengoy – Surgut, which is the only mode of transport of mineral resources on the Surgut CSP not equipped with heaters and appropriate insulation coating. In this regard, securing a reliable supply aggravated liquid feedstock to the processing power is today an urgent task.
1. Physicochemical principles of process of paraffin formation and sedimentation
Parafinnisation of equipment probable across the all technological chain.
At a low temperature separation is evident in the increasing separation temperature, increasing the pressure drop in the heat exchangers, reducing their coefficient of heat transfer and the deterioration of the quality of marketable gas [2].
Analysis of available data suggests that the concentration of fractions boiling at temperatures of 253 °C and above, in the liquid phase at a level less than 1.0 % by weight paraffin sediment on the surface of heat exchange equipment does not happen [3].
The term paraffin
is used in different meanings:
- if it is a hydrocarbon feedstock or product, the term
paraffin content
is a mixture of solid macromolecular alkanes of normal structure; - in descriptions of the processing or transport of hydrocarbons mainly meant that the stream has hydrocarbons in the solid state;
- in relation to paraffin sediments paraffin is often denoted hydrocarbon sediments as a whole.
With cooling the mixture of liquid hydrocarbon begin to stand microscopic solid pieces that consist of normal alkane hydrocarbons. As the temperature of the mixture of paraffin pieces continue to grow and reach a certain size, then their growth stops. With further lowering the temperature the pieces are formed of a different composition. Paraffin pieces in the liquid after cessation of growth in the volume of the solution are suspended. With further decrease in temperature at the expense of enlarging does not happen the particle aggregation.
For percolation process of paraffin sediments hydrocarbon mixture following conditions are necessary:
- stay liquid at a temperature below the point of formation of paraffin;
- positive difference between the temperature of the paraffinic liquid, and the wall temperature, which is conditioned heat transfer from the flow through wall.
Paraffin sediments happen on complex mechanism. Pieces of the paraffin attached to the wall and cooled. Happening new attachment of the paraffin pieces to the layer and growth thereon. Attaching pieces sediments to the layer happens with the capture liquid in the space between the pieces.
The consequence may be a paraffin sediments layer light paraffin piping or equipment, and substantial layer which causes significantly long clogging portions on cross section of the pipeline or the apparatus.
If the conditions of paraffin sediments in the stream are periodic, then the flow passage with the higher temperature may reduce the thickness of the layer to completely dissolve and flush liquid hydrocarbon stream.
When working in the presence of paraffin formation and water phase, can be form the stable emulsions such as condensate in water
, water in condensate
and other mixed forms. Pieces of paraffin stabilizes such emulsions [4].
Applied to technological equipment complex gas preparation unit, paraffin sediments is possible:
- in heat exchange equipment cooling gas in the gas preparation unit;
- in heat exchange equipment of ending cooling unstable condensate in condensate preparation unit.
Striving with paraffin sediments in heat exchangers is in prevention and liquidation paraffin sediments. The main methods include:
- providing conditions which exclude the formation of paraffin;
- using equipment which is resistant to dirt sediments;
- entering paraffin sediments inhibitor.
Relative liquid entrainment from first separators more than 25 g/(1000 m3) can lead to paraffin sediments in apparates. Principle of operation of separators is on figure 1.
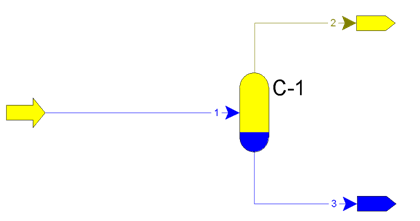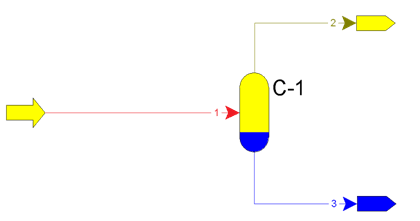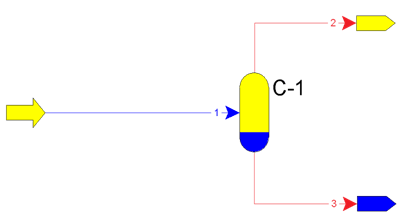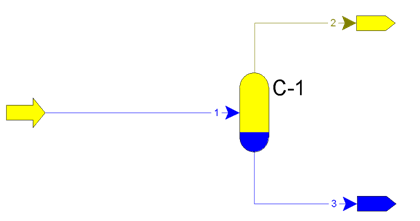
Figure 1 – Principle of operation of apparatus С-1 – flash, flow 1 – formation fluid, flow 2 – gas, flow 3 – condensate
(animation: 6 frames, 10 cycles of repeat, 27 kilobytes)
For heat exchangers of cooling unstable condensate at a temperature of commercial condensate are unavoidable conditions in education paraffin. So is used a spiral type of heat exchangers as the most resistant to dirt sediments without internal moving mechanical parts. In the spiral heat exchanger flow is always directed at an angle to the surface of heat transfer and turbulized, thereby ensuring a high heat transfer coefficient and low dirt retention surface. Figures 1 – 4 show the operation and appearance of spiral heat exchangers.

Figure 2 – The principle of operation of the apparatus
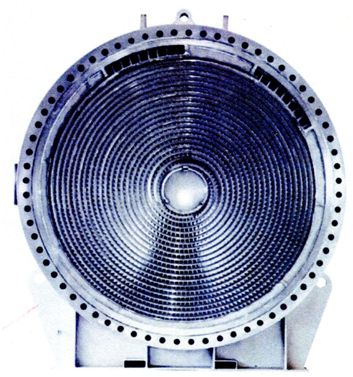
Figure 3 – View of the heat exchange channels with the cover off the apparatus
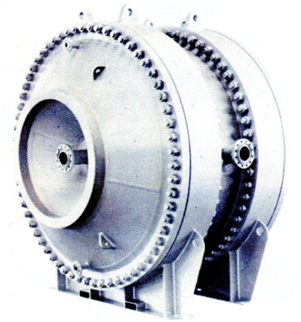
Figure 4 – Vertical mounting of the apparatus
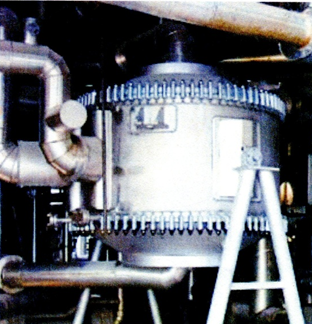
Figure 5 – Horizontal mounting of the apparatus
The main methods for the elimination of paraffin sediments in heat exchange equipment include:
- periodic mechanical purification of equipment;
- outlier physical solvent washing;
- washing with hot hydrocarbon liquid.
Washing with hot hydrocarbon liquid heat exchangers from the technology flows with the return liquid into the technological process allows removing the dissolved paraffin sediments without additional waste [4].
2. Physicochemical principles of process of hydrate formation
In developing the most gas and gas condensate fields is a problem combat the formation of hydrates. Thus the volume rate of accumulation depends on the rate of hydrate water content in gas changes with a change pressure and temperature.
Prevention of hydrate plugs is carried out support for a rather high temperature at the mouths of boreholes and at the inlet to the complex gas preparation unit. Moreover, it is assumed feed hydrate inhibitor – methanol loop beginning and in places combining gas pipelines.
Possible reasons for the formation of hydrates:
- at lower values of the gas temperature at the mouths of the boreholes;
- loops in the derivation mode at start-up and after long downtime;
- before launch loops in their full productivity, at relatively low speeds of condensate mixture;
- violating the integrity of the insulation.
Most possible locations of hydrate and paraffin-hydrate sediments:
- abrupt changes in local gas flow rate;
- in places infeed loops in gas gathering manifold;
- on the valving [2].
Gas hydrates are crystalline compounds – inclusion (clathrates) – characterized by well-defined structure for different gases. Studying the structure of gas hydrate devoted a lot of work, most of which are research B. A. Nikitin [5]. In the hydrates, the gas molecules held water molecules built from the crystal lattice.
In practical conditions production and transportation of natural gas hydrates are formed mixed, part of which may include double hydrates, large voids which are occupied by propane and isobutane, and small – methane and hydrogen sulfide, carbon dioxide, and others, as well as simple hydrates [6].
Growth form crystalline hydrates of gases quite various. It depends on the shape and composition of the gas molecules of the gas. The smaller molecular weight of hydrate-forming gas, the more straight out hydrate crystals. Methane hydrate generally has crystals which are close to linear form. Propane characterizes by a large hydrate forms of erosion. Natural gases which consist of mixtures of the individual components constitute mixed hydrates [7].
Despite their high toxicity, relatively high cost and complexity of regeneration synthetic alcohols are widely used for controlling gas hydrates. So, in 1972, the natural gas industry for the prevention hydrates spent more than 70 thousand tons of methyl alcohol that is for every thousand cubic meters of gas produced was consumed 0.3 kg of alcohol to combat hydrate formation.
Essence of process for inhibiting gas hydrate alcohols same as for inhibition electrolytes, that is the structural change ratio of pure water, and the energy of the intermolecular connections in volume of water and the transition layer at the interface of the solution – the gas and, consequently, reduces the vapor pressure of water. But the influence of alcohol is a little different from the influence of electrolytes. If at any concentration of electrolytes reduces the temperature of hydrate formation, the alcohols at certain pressures and low concentrations increase the temperature of hydrate formation, but at high concentrations – it reduces (figures 6 and 7). Probably comes the partial substitution of voids in the structure of water radical CH3, while reinforcing the clathrate formation with gas molecules neighboring vacant void. It is known that the growth of hydrate chain shall facilitate the organization of ice-like structure adjacent to the organic molecule [6].
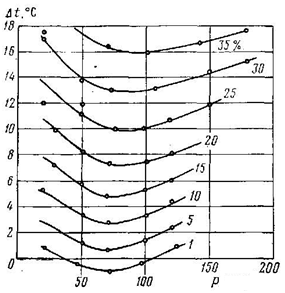
Figure 6 – Effect of the concentration of alcohol-water solutions and pressure on reduction temperature of the methane hydrate formation
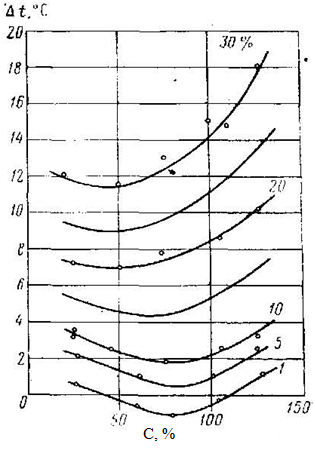
Figure 7 – Effect of the concentration of alcohol-water solutions and pressure on reduction temperature of the natural gas hydrate formation
With increasing concentration of alcohol in water there is a violation of structural organization of the water and the clathrate-forming aggregates and, consequently, reducing the likelihood of hydrate. With increasing concentration of alcohol an alcohol solution structure close to the structure of the most fortified water itself. Thus one molecule of the alcohol is surrounded by four water molecules. This assumption is supported by a certain speed of sound, mixing heat, adiabatic compressibility solutions.
The discrepancy between the effect of reducing the temperature to gas hydrate unequal composition and different pressures emphasizes the fact of differing structural changes of a water – gas in the presence of the third component (inhibitor). This fact is consistent with the change in the solubility of gases in water at variable pressures [6].
Conclusions
Based on the work performed can draw the following conclusions:
- Paraffins sediments depends on such factors:
- the lower the wall temperature the apparatus, the higher the possibility of sediment of the paraffins;
- if the fraction boiled at temperatures above 253 °C does not exceed 1 % by weight of paraffin sediment is not observed;
- the higher the speed and turbulence of the stream of hydrocarbon liquid, the less paraffins is sediment in the equipment and piping.
- When dealing with paraffin sediments should be used a range of methods:
- entry inhibitors, paraffin and / or paraffin formation;
- thermal insulation and heating pipelines;
- use of spiral heat exchangers for the ending cooling of unstable condensate;
- use of qualitative methods of separating the formation fluid.
- The use of spiral heat exchangers in shell and tube compared with the decreases compared with the number of devices and their resolution.
References
- Юнусов Р. Р. Совершенствование технологического процесса подготовки газа и конденсата (на примере Юраховского газоконденсатного месторождения) // Автореферат на соискание научной степени кандидата технических наук. – Уфа: 2008. – 24 с.
- Основные технические решения по обустройству установки комплексной подготовки газа ачимовских отложений Уренгойского месторождения.
- Бекиров Т. М., Ланчаков Г. А. Технология обработки газа и конденсата. М.: ООО «Недра-Бизнесцентр», 1999. – 596 с.
- Технологический регламент на проектирование установок подготовки газа и конденсата объекта «Обустройство ачимовских отложений Уренгойского месторождения Самбургского лицензионного участка на период ОПЭ».
- Сочеванов Н.Н. Количественная закономерность между упругостью водяного пара и количеством воды, сорбированной почвой. «Почвоведение», 1956. – 304 с.
- Макогон Ю. Ф. Гидраты природных газов. М.: «Недра», 1974. – 208 с.
- Макогон Ю. Ф. Кристаллы гидратов газов. «Нефть и газ», 1970.
