Contents
- Introduction
- 1. Theme urgency
- 2. Goal and tasks of the research
- 3. Methods of detection and evaluation of grain size
- 4.Study of grain growth kinetics of solid solutions under heating
- Conclusion
- References
Introduction
In modern engineering there is a need for materials with a high level of mechanical properties, which are determined by the chemical composition and structure. The basic parameter of the structure is the size of austenitic grain. [1]
Stainless steels are a large group of chromium, chromium-Nickel and chromium-manganese-Nickel steels with a content of more than 12 % Cr, preserving light metallic luster under the influence of the atmosphere, i.e. stainless properties. Chromium increases the corrosion resistance of steels also in other environments, mainly oxidizing, which is widely used in the manufacture of chemical equipment.[2]
The higher the chromium content in steel, the higher their corrosion resistance in atmospheric conditions and in a number of corrosive environments. In addition to chromium, Nickel, manganese, carbon, molybdenum, tungsten, niobium and other elements are introduced into the steel to give them special properties and structure. Stainless and corrosion-resistant steels are widely used in the chemical industry in the manufacture of various chemical equipment, in the oil industry. In the metallurgical and machine-building industries heat-resistant steels and alloys are used in the manufacture of elements of furnace equipment.[2]
Corrosion-resistant and heat-resistant steel and alloys based on iron and Nickel-one of the most important classes of special structural materials used in most important industries: chemical, thermal and nuclear power, pulp and paper, oil and gas, medical, shipbuilding, automotive, food, household appliances, industrial and civil construction, etc.
A distinctive feature of corrosion-resistant steels and alloys is their increased resistance to uniform corrosion in a wide range of corrosive environments of varying degrees of aggressiveness. Along with this, many of them are resistant to local types of corrosion (intergranular, pitting, slit, corrosion cracking) in halide-containing environments and have a high level of physical and mechanical properties. Heat-resistant (scale-resistant) steels and alloys are characterized by high resistance against chemical destruction of the surface and in gaseous media at temperatures above 550°C, operating in the unloaded and underloaded States. [3]
1. Theme urgency
The purpose and relevance of the work is to obtain more data on the influence of heating parameters on the size of the original and actual grain austenite austenite steel and ferritic classes, as well as to study the processes of gas corrosion.
2. Goal and tasks of the research
The objectives of the study included the search for methods to identify the structure of austenitic and ferritic steels, the study of the structure of these steels, after treatment at different temperatures and holding times, as well as the study of the propensity to gas corrosion of steels of different structural classes.
3. Methods of detection and evaluation of grain size
The process of manufacturing metallographic grinding usually includes the following basic operations: 1) cutting the sample and surface preparation; 2) grinding; 3) polishing; 4) etching. [4]
A well-prepared microplate must meet a number of requirements. First of all, it must be representative of the structure and properties of the object (part) under study.[5]
Cutting, grinding and polishing of the sample should be designed in such a way that its surface remained minimal layer of the deformed metal. There should be no scratches, scratches, pits and dirt on the surface of the strip. In the course of preparation of the loop there should be no painting of nonmetallic inclusions of carbide and other phases. In addition, the surface of the strip should be flat enough to be considered at high magnification. Cutting on samples was carried out with an abrasive wheel. Sample sizes - steel 08×17-25×12×1,5. Samples after cutting for the manufacture of thin sections were placed in a clamp.[6]
After obtaining a flat surface, the samples were sanded with paper grinding sandpaper of different grit.
Polishing is used to remove small scratches left after grinding and to obtain a smooth mirror surface of the strip. Polishing was carried out on a rotating circle with the tense of the polishing material In an abrasive used chromium oxide. The surface of the sample was polished on a polishing wheel using cloth. After polishing, the sample was washed with water, degreased with a swab soaked in alcohol, and dried with filter paper. Further, the polished surface of the sample was subjected to etching. After viewing the non-etched plume for a more complete study of the structure of the alloy, the plume is etched. [7]
There are several methods of etching, differing in effect on the surface of the metal. [8]
To identify the structure of steels that are corrosion resistant, the following etchants were tested:
Etchant ¹1-Hydrochloric acid 100 ml, sulfuric acid 10 ml, copper sulfate 20 g, hydrogen peroxide 2 ml, water 100 ml. hydrogen Peroxide is added immediately before etching [14].
Etchant ¹2 - "Royal vodka" (3 volume HNO3+1 volume HCl) [14].
Ruler ¹3-Saturated aqueous solution of picric acid with the addition of surfactants (liquid soap, shampoo, detergent Gala)
Etchant ¹4 -50% H2O+50% HNO3 [9].
In the experiment were chosen the following etchants: -for steel 10H18N10T- "Aqua Regia" -for steel 08Õ17 - Etchant No. 1
Microstructure studies were performed using metallographic microscopes "Neophot 21" and MIM-7. The average grain size was determined using photographs of microstructures taken at 500 and 200 times magnification using the secant method.
4. Study of grain growth kinetics of solid solutions under heating
Figure 4.1 presents a graph of the average grain size of austenite from the holding time at a heating temperature of 900°C
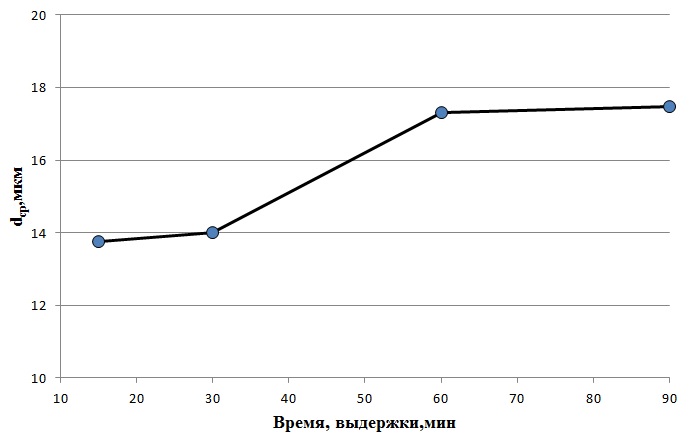
Figure 4.1 - graph of the average grain size of austenite from the holding time at a heating temperature of 900°C
Figure 4.2 shows the dependence of the average grain size of austenite on the holding time at a heating temperature of 1000°C
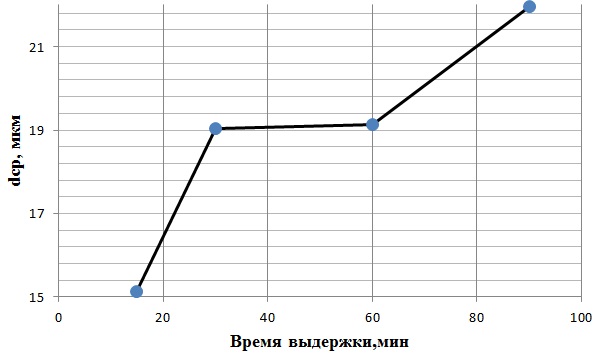
Figure 4.2 - graph of the average grain size of austenite from the holding time at a heating temperature of 1000°C
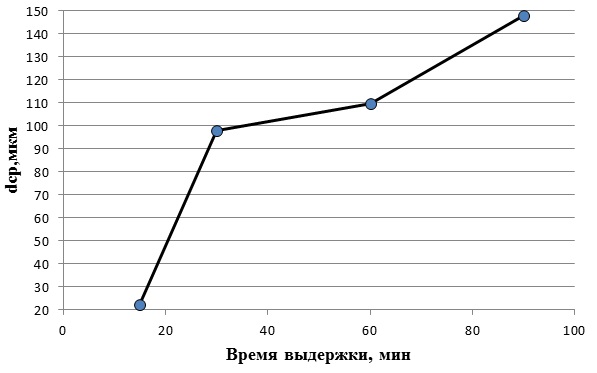
Figure 4.3 shows the dependence of the average grain size of austenite on the holding time at a heating temperature of 1100°C
Conclusion
Based on figure 4.1, it can be concluded that at a heating temperature of 900°C, the structure of 08X17 steel has a uniform appearance, the austenite grain does not grow significantly over different aging times. Some increase in grain is noticeable after 30 minutes of aging, grain size varies from 14 microns to 17 microns. After 60 minutes of exposure on the structure noticeable beginning of collective recrystallization.
Based on figure 4.2, it can be concluded that at a heating temperature of 1000°C in the structure of steel 08X17 recrystallization begins, the grain after 15 minutes of aging begins to grow, but intensive grain growth is not observed. During the exposure of 30..90 minutes grain size varies by a few microns, from 19 microns with a holding time of 30 minutes to 22 microns after 90 minutes.
Based on figure 4.3, it can be concluded that at a heating temperature of 1100°C and a shutter speed of 15 min austenite grain in steel 08X17 still remains small-22 microns. But with the increase in the duration of exposure at this temperature begins a noticeable growth of grain. Compared with the grain size after 15 minutes, at 30 minutes it increased more than 4 times, to 98 microns. After 30 minutes austenitic grain began to grow rapidly. As a result, after aging in 90 minutes, a grain size of 148 microns was obtained.
References
- Goldstein, M. I., Grachev S., Wexler YG Special steel / - M.: metallurgy, 1985. - 408 p.
- Solncev, Y. P., Pryakhin E. I. Science/ St.-Petersburg: Khimizdat, 2007. - 784 p.
- V. G. Sorokin, A. V. Volosnikova, S. A. Vyatkin, and others; ed. by V. G. Sorokin./ Marochnik of steels and alloys — M.: mechanical engineering, 1989. - 640 p.
- Knorozov B. V., Usova L. F., Tret'yakov A. V., etc. the Technology of metals and materials. 1987.- 800 p.
- Lakhtin Yu. M. foundations of physics. Textbook for colleges. 1988.-319 p
- Lakhtin Yu. M., Leontieva, V. P. materials Science: Textbook for higher technical schools. - 3rd ed., pererab. I DOP. - M.: mechanical engineering, 1990. - 528 p.
- GOST 5639-82 of Steel and alloys. Methods of detection and determination of grain size
- M. L. Bernstein, A. G. Rakhshtadt. Metallurgy and heat treatment vol. 1, publishing house "metallurgy", Moscow, 1995, 304 p.