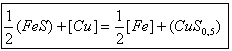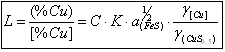|
Dissertation
Introduction
In modern metallurgy quality of scrap-metal is constantly worsened due
to the admixtures of a color metals it results the considerable variations of
the copper consistence in the metal. For example, the consistence of the cop7per in a metal which is produced by a remelt in arc furnace
of the classified scrap of the different classes and it hesitates from 0,06 to
0,54%. Increasing requirements of the steel’s quality create the problem
of copper removing very actual.
A copper worsens a cold and hot deformation and so enhances the degree
of crackdeeps. [2, p.14]. Among other colour admixtures a copper is one of most
problem of the refining it contingently that a copper as compared to iron is
more noble element, that does not allow to utilize the traditional methods of
refining [4, с.31]. Its concentration in a scrap can reach to 0,5% and more.
Thus, for the guarantee of the required quality level of the metal product the consistence of the
copper in steel must not exceed 0,2%[3, p.87].
Thus, removing of the copper and tin from a offcuts are aimed for the
useing of muddy and cheap scrap steel to make more high grade of the steel [7].
In the conditions of electrofusion all processes of refining have
oxidizing character and such as a copper has a less chemical similar to oxygen
the iron then removing of the copper is impossible.
The modern industry have the following methods of utillize:
1. The control of the scrab quality by sorting and separation of it
(magnetic separation of the crushed scrab, cuprum smelting, anodal dissolution of the scrab and other).
2.The method of the vacuum evaporation with gas stirring.
3. The refining of the iron fusion
from a copper by sulphide slags on the basis of different
sulfur-containing connections of a sodium (Na2S, Na2SO4 and other).
4.The method of a cast-iron dilution.
5.A neutralization of the copper in steel by additing of elements.
6. The removing of the copper by a filtration
of fusion through ceramic filters.
Aims and tasks
The purpose of research conducted in this work is development of
effective method of copper removing from the iron-carbon fusion by extraction
of it in a sulphide phase.
For achievement of the put
purpose it is necessary to execute the followings tasks:
1)
to investigate the terms of
copper transfer from the iron-carbon fusions in a sulphide phase;
2)
in experimental terms to research
and realization the features of
sulphidizing method
3)
to develop the
technological receptions of effective of
the refining at the iron-carbon fusion and it applies to the concrete
industrial terms.
Teoretical part
The organization of the removing of
copper from the iron-carbon fusions
has next circumstance, the sulphur possesses is very active to the copper then
for the iron.
The chemical reaction of the copper removing from a liquid metal to a
sulphide phase can be presented as follows:
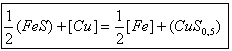 (1)
(1)
From
the equalization of the balance constant this reaction we can obtaine the expression of the distributing
coefficient of the copper:
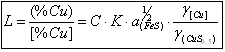 (2)
(2)
where C - the concentration recounting coefficient from a molecular to
the mass percents;
K- to is a constant of equilibrium of reaction;
 - a
activity of component i; - a
activity of component i;
 - a
coefficient of activity of component i. - a
coefficient of activity of component i.
For the increase of the distributing coefficient of
copper occur due to high activity of sulphide of iron and high coefficient of
copper activity in a liquid metal, and also the low coefficient of activity of
copper’s sulfide in a slag. It is accepted on well-known sources, that the
coefficient of distributing of copper due to the using of slags on the basis of
sulfide of iron matters about 9, and at addition of sulphides of alkaline
metals (in particular NAS) allows to promote it in 3 times.
The treatment it by the sulfide slags a degree of copper removing is
67-68% in the interval of temperatures of 1673-1773К in
the flow of 6 min [6, p.31] and during treatment the concentration increases in
fusion in 2-3 times.[3, p.88].
At addition of sulfide of aluminium a distributing coefficient increases
to 30 [9].
Experimental part
Experiments have made in the laboratory terms of problem laboratory at
department «Electrometallurgy» in Tamman’s furnace and in experimentally-industrial terms on OAA
Konstantinovskiy factory of “Vtormet” in a 200 kg induction furnace
too.
List of literature
1.
Perspective methods of
delete of admixtures of the coloured metals from iron-carbon расплавов/
V.A.Kudrin/in-t «Chermetinformaciya» M., 1992 (Obzorn. Inform. Grey.
Steel-smelting production. Vyp. 1. 26 p.).
2.
Problems of delete of
copper from steel // «Steel».№7.1991г.
3.
Y.V. Kosteckiy of Prospect
of the use of sulfides for the affinage of iron-carbon fusions from a cut-in
copper // «Metal and casting of Ukraine».
№3-4, 2005г.
4.
A.I. Hare and other
Analysis of reality of technology of delete of copper from liquid iron, built
on evaporation // «Electrometallurgy». №10, 2003г.
5.
Problems of delete of
copper from steel // «Steel».№7.1991г.
6.
V.I. Kashin and other
Fiziko is chemical conformities to the law of co-operation of copper and
sulphur in fusion of iron at treatment a sulfide slag // «Steel». №3. 1991г.
7.
“Copper and Tin in Steel Scrap
Recycling” / Luben Savov, Elena Volkova, Dieter Janke // RMZ - Materials and
Geoenvironment, Vol. 50, No. 3, pp. 627-640, 2003
8.
Wang, C.; Himara, J.; Nagasaka, T., Ban-Ya, S.
“Copper Distribution between FeS-Alkaline or – Alkaline Earth Metal Sulfide Fluxes
and Carbon Saturated Iron Melt”, ISIJ International 1991, Vol.31, 11,
1309-1315.
9.
Shimpo, R.; Fukaya, Y., Ishikawa,
T., Ogawa, O. “Copper Removal from Carbon-Saturated Molten Iron with Al2S3-FeS Flux”,
Metallurgical and Materials Transactions B 1997, 28B, 1029-1037.
|




 serega_pavlenko@mail.ru
serega_pavlenko@mail.ru