

 |
 Faculty: Environment and Chemical TechnologySpeciality: Chemical technology of high molecular connectionsTheme of master's work:Research of properties of permissible explosivesScientific adviser: Mangos Juriy |
| |
Abstract IntroductionContinuous development of industry requires a steady increase in coal production, because it is main type of energy and chemical raw materials. Blasting operations are used for coal production. Explosions of methane in coal mines have been observed starting from the XVIII century. Because mined beds occur at a great depth and have a high volume of gas. Coal seams are bedded a depth greater than 720 m in the mines of the Donbass. Approximately 90% of mines in Ukraine are gas.Only permissible explosives are used in coal mines dangerous on gas and slack explosion. The use of permissible explosives V and VI classes is significantly reduced in recent years. Sales volume of permissible explosives V classes came down in three times more. The recession in demand is related to the high cost and low efficiency in blasting. The available task is development of new mixtures of permissible explosives. They will posses high safety properties and relatively low cost price. The purpose of my work is to study the ion-exchange of explosives on the basis of ammonium sulfate. The new permissible explosiveThe reaction between sodium nitrate with ammonium chloride is basis of ion exchange process, which was proposed by Bihel in 1902. In 2003, Zenin proposed to use as ion pairs of sodium nitrate and ammonium sulfate. A prototype of the explosive was based on uglenit P52. Its choice is based on the following information:
Table 1
The calculation of energy characteristicsThe calculation of energy characteristics was performed on a computer, by meansof Mathcad. the calculation explosive transformation heat, the temperature of the explosion, the total ideal of the explosion, efficiency of the explosion and the pressure of explosion products, equations of explosive transformation are presented in the program. Equations of explosive transformation for permissible explosives V classes were obtained after the calculation.The reaction of explosive transformation for uglenit 13P is 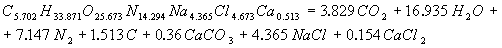 The reaction of explosive transformation for uglenit P52 is 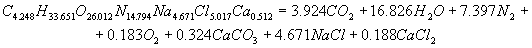 The reaction of explosive transformation for prototype is 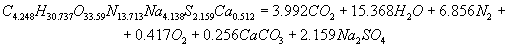 The reaction of explosive transformation for uglenit 10P is 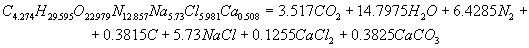 The calculated energy parameters PE shown in Table 2 Table 2
Tests on safety properties uglenit P52 and prototype were carried out on the plant Petrovsky. Critical mass of an explosive, which no inflammation of the methane-air mixture was determined. For uglenita P52, this mass is 300 g, for a prototype of the mass of the limiting charge no less than uglenit 10P. The deductions were taken out after conducted work.
Subsequent work will be directed on determination components ratio optimum of new explosive.  |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||