Abstract
Contents
- Introduction
- 1. Importance, purpose and objectives of the study
- 2. General characteristics of clays
- 3. Physical and chemical basis of the adsorption process
- 4. Experimental part
- Conclusion
- References
Introduction
Among many factors forming health the most important one is environmental factor. Industrial human activities have led to water pollution by harmful substances: metals in ionic form, petroleum products, toxic synthetic substances and other pollutants [1].
Adsorption method is the simplest, least expensive, affordable and effective among all special methods of water treatment. [2]
Adsorption processes using natural mineral adsorbents are used more and more because of the possibility of their use in water treatment processes due to their low cost. Overview periodicals sorption processes in natural minerals showed that these processes are poorly understood, and this area requires more detailed study.
A long time use of classic water treatment technologies have led to increased pollution of the hydrosphere more that requires new strategies and technologies in water treatment [3].
1. Importance, purpose and objectives of the study
An activated carbon is used as an adsorbent in adsorption water treatment methods. But it’s appropriate and effective use of natural sorbents, which are inexpensive and available materials on one hand, and on the other - can achieve a sufficient degree of purification. Such sorbents are used due to their relatively high capacitance, ion exchanging properties of some sorbents, low cost and availability. Topicality is the use of natural sorbents - clays [1].
The purpose of the work was to study the sorption properties of some Donbass clays. To do this, six samples from different deposits of clays have been elected: clay loam (Belaya Balka field), two samples of clay, which differ in the content of aluminum, from Chasov-Yarsk field, clay deposits of Lugansk field, two samples from Konstantinovka clay field, which differ in the content of titanium and iron.
To achieve the goal the following objectives have been formed:
– studying the chemical composition of the clays;
– experimental investigation of the clays sorption activity according to standard procedures and determination the iodine number;
– constructing of the adsorption isotherm;
– definition the sorption properties of clays relatively different substances.
2. General characteristics of clays
Argillaceous rocks are the most common inorganic sorbents for water treatment. They have developed structure with micropores, which have different sizes depending on the type of material. Sorption mechanism of water pollution on clay minerals is rather complex and includes the Van der Waals interaction between the hydrocarbon chains and silicates microcrystals developed surface and Coulomb interaction of charged and polarized molecules of sorbate with positively charged areas of the surface of the sorbent containing ions H+ and Al3+ [4].
Adding clay minerals adsorbents during waste and natural waters treatment on the settling will not only allow to get rid of the dangerous anthropogenic contaminants by adsorption without chemicals, but also improve the structure and mineralization of water [6] .
Advantages of using such adsorption materials are the following:
– natural sorbents are widespread in Ukraine [7];
– natural sorbents are available and inexpensive material [7];
– the structure of the sorbent is not destroyed (as compared with the polymer, synthetic foam absorbents ) by reacting with oil [3];
– a wide range of adsorbed substances (oil, heavy metals, pesticides, radionuclides) [3];
– clays do not burn unlike synthetic foam, nonwoven sorbents [3];
– operable in a wide temperature range from -20 to +100 0C;
– adsorption technologies using natural dispersed sorbents provide a sufficient degree of purification [7];
Thus, the clays of Donbass fields would be used as adsorbent for the extraction from natural and waste waters of various pollutants.
3. Physical and chemical basis of the adsorption process
Adsorption waste water treatment is widely used in various industries. This is one of the effective ways of deep natural and waste waters from organic substances, heavy metals, petroleum products.
Sorption is effective for decolorization and eliminates odors and flavors from natural waters. The adsorption can be used to treat waste water from phenols, herbicides, pesticides, aromatic nitrocompounds, surfactants, dyes and other dissolved substances contained in the waste water.
The advantage of the method is its high efficiency, the possibility of treatment waste water containing multiple contaminants, as well as recovery of these substances. The efficiency of sorptive purification is about of 80-95 %, depending on the chemical nature of the sorbent, the value of adsorption surface and of its availability, the chemical nature of the contaminant and its solution state [4].
Basic information on the sorption properties of the material and the nature of adsorption of certain substances may be obtained from the adsorption isotherms of characterizing the dependence of the concentration of the sorptive capacity (or pressure) component sorbed, at a constant temperature. Brunauer, Emmett and Teller [8] identified five major types of sorption isotherms, the so-called BET isotherms (Figure 3.1). Convex portions isotherms I, II and IV indicate the presence of micropores in the adsorbents, but moreover, sorbents II and IV also have macroporosity. Isotherm types III and V are less frequent and describe the strong intermolecular interaction in matter sorbate. The steepness of the isotherm type I describe the size of micropores sorbents: a - ultramikroporistih b - microporous.

Figure 3.1 — Types of adsorption isotherms by BET
In the intermediate equilibrium concentrations (in small areas of change in the concentration of the adsorbate), the dependence of adsorption the concentration can often be described by the Freundlich equation, which is based on the assumption that the adsorption isotherm is a parabola. In logarifmovannomu form of Freundlich equation is straightforward.

Figure 3.2 — Scheme of determination of the constants in the equation Frendliha
where к и 1/n – constants.
Thus, a complex heterogeneous adsorption process whose efficiency depends on many factors.
4. Experimental part
To confirm the possibility of use as sorbent clays , their tests were carried out by standard methods [9,10].
The sorptive activity can be evaluated based on the iodine number , which is defined based on a measurement of initial and residual iodine concentration in the feed solution after adsorption and defining titrimetrically .
For this sample clay fraction of 1 mm and a mass of 1.00 g were placed in a conical flask of 250 cm3 was added thereto by 100 cm3 of 0.1 N iodine solution in potassium iodide . Then the clay is separated from the solution and measured the initial and residual iodine concentration in the feed solution and the solution after adsorption titrimetric method. Treatment results were evaluated by the formula ,%:

where V1 - volume of sodium thiosulfate (0.1 N), which went to 10 cm3 of titration of iodine in potassium iodide, cm3;
V2 - volume of sodium thiosulfate (0.1 N), which went to 10 cm3 of titration of iodine in potassium iodide, after treatment with clay, cm3;
0.0127 - mass of iodine, which corresponds to 1 cm3 of sodium thiosulfate, g;
100 - the amount of iodine in potassium iodide, which took lightening clay cm3;
m - sample weight of clay, 1.00 g
The results are presented in Table 4.1.

Рисунок 3.5 — Sorptive activity evaluated based on the iodine number
(animation: 2 frames, 7 reiterations, 154 kilobytes)
Table 4.1 — Results of calculation
|
|
Iodine number, % |
|
Loam (Belaya Balka) |
12,7 |
|
Chasov-Yarsk field, II grade |
38,1 |
|
Lugansk field |
31,8 |
|
Chasov-Yarsk field |
57,2 |
|
Konstantinovka clay field |
38,1 |
|
38,1 |
An important characteristic is the definition of sorbents for dye adsorption activity. According to [10] determined the adsorption activity with respect to methylene blue, methyl orange, and methyl red.
For this purpose a sample of coal weight 0,300 g was placed in a conical flask of 100 cm3 25 cm3 of the solution added to the dye. Thereafter, the optical density at photoelectrocolorimeter with a blue filter, and a wavelength of 400 nm in cuvette, with the distance between the working faces 10 mm. As a control, distilled water solution was used. According to the obtained absorbance based on the calibration curve was determined by the residual concentration of the dye.
Adsorptive activity was calculated by the formula:
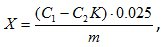
where C1 – concentration of the dye stock solution, mg/dm3;
C2 – the concentration of the dye solution after contact with activated carbon, mg/dm3;
K – coefficient of dilution;
m – mass of activated carbon sample, g;
0,025 – the amount of the dye solution, participating in the study, dm3.
The results of studies of the adsorption capacity shown in Tables 4.2–4.3.
Table 4.2 — Results of determination the adsorption capacity of clays in methyl orange solution
| Sorbent | Initial concentration Свх, mg / dm3 | Hitch clay m, g | Residual concentration, Сзал, mg / dm3 | Adsorption capacity g, mg/g |
| Loam Belaya Balka | 1500 | 0,30 | 2,1 | 108 |
| Chasov-Yarsk field, II grade | 1500 | 0,30 | 2,1 | 108 |
| Lugansk field | 1500 | 0,30 | 2,4 | 105 |
| Chasov-Yarsk field | 1500 | 0,30 | 1,2 | 108 |
| Konstantinovka clay field | 1500 | 0,30 | 4,3 | 96 |
| Konstantinovka clay field | 1500 | 0,30 | 3,3 | 98 |
Table 4.3 — Results of determination the adsorption capacity of clays in methylene blue solution
| Sorbent | Initial concentration Свх, mg / dm3 | Hitch clay m, g | Residual concentration, Сзал, mg / dm3 | Adsorption capacity g, mg/g |
| Loam Belaya Balka | 1500 | 0,30 | 0.6 | 125 |
| Chasov-Yarsk field, II grade | 1500 | 0,30 | 0.8 | 124 |
| Lugansk field | 1500 | 0,30 | 2,4 | 123 |
| Chasov-Yarsk field | 1500 | 0,30 | 1,2 | 114 |
| Konstantinovka clay field | 1500 | 0,30 | 1,5 | 124 |
| Konstantinovka clay field | 1500 | 0,30 | 1,7 | 124 |
Table 4.4 — Results of determination the adsorption capacity of clays in methyl rad solution
| Sorbent | Initial concentration Свх, mg / dm3 | Hitch clay m, g | Residual concentration, Сзал, mg / dm3 | Adsorption capacity g, mg/g |
| Loam Belaya Balka | 1500 | 0,30 | 3,8 | 93 |
| Chasov-Yarsk field, II grade | 1500 | 0,30 | 3,2 | 98 |
| Lugansk field | 1500 | 0,30 | 3,3 | 98 |
| Chasov-Yarsk field | 1500 | 0,30 | 3,2 | 98 |
| Konstantinovka clay field | 1500 | 0,30 | 4,3 | 89 |
| Konstantinovka clay field | 1500 | 0,30 | 3,3 | 98 |
Conclusion
The results of the master's work are the following:
a) considered a general characteristic of clays;
b) considered the physical and chemical basis of the adsorption process, the basic equations and evaluated factors affecting the adsorption process are shown;
c) iodine number of clays was experimentally determined. Iodine number differs considerably due to the different mechanisms of sorption on clays.
d) sorption properties of clays have been experimentally studied in relation to dyes. As the research results, all samples of clays have sorption properties. The magnitude of the sorption capacity of clay is 50–60 % of a similar magnitude for the active carbons.
In the future we plan to evaluate the mechanism of adsorption on clays based on a study of the adsorption isotherms for dyes as well as explore the adsorption properties of clays in relation to the different nature pollutants (heavy metals, organic compounds, etc.).
References
- Везенцев, А.И. Адсорбционные свойства продуктов обогащения природных монтмориллонитсодержащих глин // А.И. Везенцев. – Белгород: Белгородский государственный национальный исследовательский университет, 2011. – С. 103-108
- Івченко, В.Д. Очищення стічних вод від іонів амонію та феруму глинистими мінералами Сумської області: Автореф. канд. техн. наук: 21.06.01 / Сумський національний аграрний університет – С., 2012. – 35 с.
- Ланець, Г. І. Дослідження адсорбційних властивостей глин родовищ Донбасу / О.А. Трошина, Г.І. Ланець // Матерiали XXIV Всеукраїнської наукової конференцiї аспірантів і студентів. – Т.1 – Донецьк: ДонНТУ, 2014. – С. 92 – 94
- Ланець, Г. І. Адсорбційні властивості глин родовищ Донбасу / О.А. Трошина, Г.І. Ланець // Матерiали ХХII Міжнародної науково-технічної конференцiї. – Харків: ХГТУСА, 2014. – С. 71
- Запольський, А.К. Водопостачання, водовідведення та якість води: Підручник / А. К. Запольський. – К.: Вища школа, 2005. – 671 с.
- Тарасевич, Ю.И., Овчаренко, Ф.Д. Адсорбция на глинистых минералах / Ю.И. Тарасевич, Ф.Д. Овчаренко . – К.: Наукова думка, 1975. – 340 с.
- Смирнов, А.Д. Сорбционная очистка воды / А.Д. Смирнов. – Л.: Химия, 1982. – 168 с.
- Фізико-хімічні основи технології очищення стічних вод / За редакцією А.К. Запольського. - Київ: Лібра, 2000. – 549 с.
- ГОСТ 6217 – 74. Уголь активный древесный дробленый. – М.: Государственный комитет стандартов, 1974. – 6 с.
- ГОСТ 4453 – 74. Уголь активный осветляющий древесный порошкообразный. Технические условия. М.: Государственный комитет стандартов, 1974. –11 с.


