Abstract
Contents
- Introduction
- 1 An analitical review of the present methods of defenolation
- 1.1 The steam method of the defenolation
- 1.2 The extraction methods of cleaning
- 1.3 The biochemical purification methods
- 2 The synthesis of sulphochlorides of anilides of sulphoacids with screened sulfonilamide substituents
- 2.1 Synthesis of N–benzensulphonyl–2,4,6–trimethylaniline
- 2.2 The alkylation of benzensulphonyl–N–2,4,6–trimethylaniline
- 2.3 Sulphochlorination of N–methyl–N–benzensulphonyl–2,4,6–trimethylaniline
- 2.4 The cleaning of sulphochlorides of anilides of sulphoacids
- 2.5 Determining the structure of the synthesized compounds
- 3 The kinetic study the of neutral hydrolysis of 3–N(XАrSO2)–NMe]–2,4,6–Me3–C6H2SO2Cl
- 4 The influence of the structural factors of the substituents on the character of the neutral hydrolysis in 70% a.d.
- 5 The development of the technological scheme of the chemical wastewater defenolation
- Conclusion
- References
Introduction
Actuality:
The work is devoted to the chemical extraction of phenols from the industrial wastewater with using of the derivatives of arenesulphoacids. The method is more economical in comparison with the most popular. The method of chemical defenolation is independent from the concentration of phenolic effluent and may be use practically on all enterprises without limits. In addition, the extracted phenols and their derivatives can be used in various industries as chemical reagents, biologically active maters and others [1,2].
Purpose, the main tasks, possible practical results, scientific novelty
Purpose: the definition of nature of the positive steric effect at sulphonic centre in steric hindered sulphochlorides of anilides of sulphoacids in the nucleofilic substitution and the development of the technological scheme of the chemical defenolation wastewater on this base.
The main tasks:
- The kinetic study of 3–[N(ХArSO2)–NMe]–2,4,6–Me3–С6НSO2Cl in 70 % aqueous dioxane (a.d.) at 303–323 K.
- The calculation of the thermodynamic parameters of the transition state for substitution at the sulphonyl center.
- Explanation of the mechanism of the nucleophilic substitution hydrolysis conditions.
- Justification and development of the technological scheme of the chemical defenolation of wastewater on the investigated substrates.
Object of research: steric hindered sulphochorides of anilides of sulphoacids
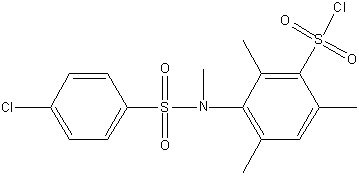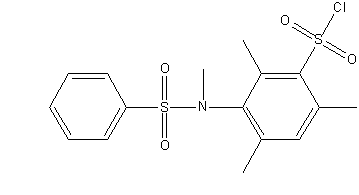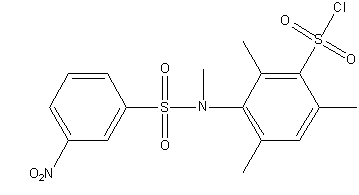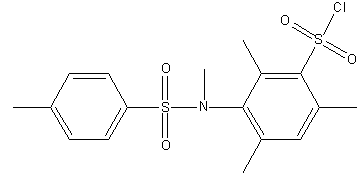
Figure 1 – Steric hindered sulphochorides of anilides of sulphoacids
(gif–animation: 4 frames, 5 repeats, 5.45 kB)
Possible practical results: justification and development of the chemical defenolation of industrial water.
Scientific novelty:
- The synthesis of the compounds 3–[N(ХArSO2)–NMe]–2,4,6–Me3–С6НSO2Cl, which are underscribed in the literature.
- The study of the substrates reactivity in neutral hydrolysis.
- Identification of the isoparametric field on temperature and the increasing of the possibilities of the defenolation on this base.
- The development and detailing of the technological scheme of the chemical defenolation.
1. An analitical review of the present methods of defenolation
1.1 The steam method of the defenolation
The steam method of defenolation is combined with wet quenching of coke and a closed cycle of phenolic waters, or the biological refining. It is used on domestic coke plants. The defenolation degree is at least 85%, and the remain content of phenol in the treated water — no more than 0.2 g/l [3].
This method consist of: the blowing of phenols of the water steam from hot wastewater and passing of this mixture through the absorption alkaline solution for formation of the phenolates.
Advantages: compact installation, ease of processing, the ability to automate
Disadvantages of the method: the large energy consumption, the inability to completely remove phenols.
1.2 The extraction methods of cleaning
Extraction methods are suitable for the extraction of phenols from water with concntration more than 2 g/l phenols.
This method conclude of: the addition immescible solvent to phenol water and the formation of bilayer mixture of defenolated water and solvent with phenols. Then phenols is extracting from phenol mixture by solvent distillation or as the phenolates [3,4].
The Advantages of this method: the high degree of defenolation, more complete extraction of phenols and almost complete — oils and resins than in previous method.
Disadvantages: high cost and the necessity of the solvent regeneration, the complexity of the technological scheme.
1.3 The biochemical purification methods
Biochemical methods are based on the ability of microorganisms to oxidize organic compounds. The final products of biodegradation of impurities in the wastewater are carbon dioxide (CO2) and water. These methods provide a deep cleaning of wastewater by the phenol decomposition. There are two types of microorganisms: activated sludge (biofilm) and specific culture bacteria for destruction of the components of wastewater. The best results are obtained after prior dilution of phenolic wastewater by technical or industrial water. [3,5].
Advantages of the method: deep purification of waste water.
Disadvantages: high cost of exploitation, the equipment complexity, the using only for dilute wastewater and the small work temperature interval.
2 The synthesis of sulphochlorides of anilides of sulphoacids with screened sulfonilamide substituents
The syntesis of steric hindered derivatives of this class is very difficult. During the synthesis by [6] there are many compounds in reaction mixture as a result of isomerisation and decomposition of initial substrates. Synthesis of such structures is complicated by the fact that under the action of sulphate, chlorosulfonic acid and hydrochloric acid, are in the reaction mixture the desired isomerized substrates are decomposed into several groups derivatives: salts, benzenesulphochlorides, sulphonated secondary amines, etc. It appears that this is due to the almost complete absence of literature information about obtaining these compounds.
The total number of compounds which underscribed in literature is 12.
2.1 Synthesis of N–benzensulphonyl–2,4,6–trimethylaniline
Synthesis was carried out by reacting:
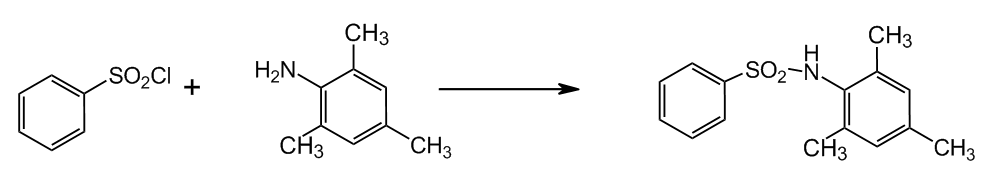
Figure 2 — Synthesis of N–benzensulphonyl–2,4,6–trimethylaniline
Reagents:
- benzensulphohloride — 4,41 g (0,025 mole).
- 2,4,6–trimethylaniline — 4,05 g 0,030 mole).
- Sodium carbonate — 2,12 g.
Benzenesulphochloride and aniline are mixed in a conical flask volume 200 ml then added water to 50 ml, and small portions - ash by heating and stirring. After the reaction solution is acidified with HCl until sedimentation.
The resulting N–benzensulfonil–2,4,6–trimethylaniline is filtered, washed with distilled water and reprecipitated from alkali. The filter residue is dried in air.
Yield 5.2 g (0.02 mol) — 80%.
2.2 The alkylation of N–benzensulphonyl –2,4,6–trimethylaniline
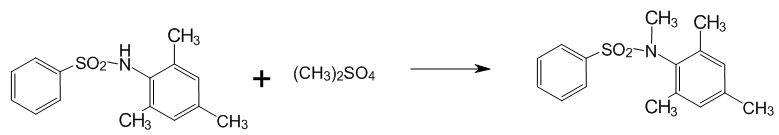
Figure 3 — The alkylation of N–benzensulphonyl –2,4,6–trimethylaniline
Reagents:
- N–benzensulphonyl–2,4,6–trimethylaniline — 7,225 г (0,025 mole).
- Alkali NaOH — 2,1 g (0,025 mole).
- Water — 50 ml.
- Dimethyl sulphate — 5,04 g (0,04 mole) 3,8 ml.
The device for syntes consist of three necked flask, stirrer and dropping funnel on an oil bath. The reaction mixture with water, 1 g alkali and sulphonylamide in flask is heated to 60 degrees under stirring until complete gomogenisation. Then it is added dimethyl sulphate (DMS) by drops though the dropping funnel. During the reaction an insoluble sediment is formed. After dropping dimetyl sulphate the sediment is filtered of, the filtrate is acidified with hydrochloric acid. The filter residue is dissolved in 15 ml acetone and poured in 50 ml 1 % alkali. To remove precursor the sediment is filtered and the filtrate acidified.
Yield 3.53 g (0.016 mol) — 63%, the reminder of initial compound is 1.3 g
2.3 Sulphochlorination of N–methyl–N–benzensulfonil–2,4,6–trimethylaniline
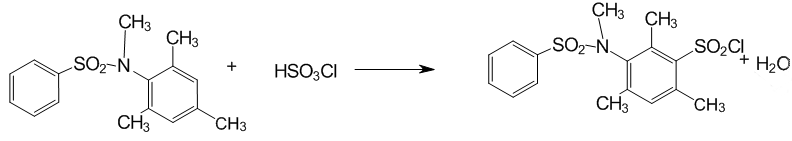
Figure 4 — Sulphochlorination of N–methyl–N–benzensulfonil–2,4,6–trimethylaniline
Reagents:
- N–methyl–N–benzensulphonyl–2,4,6–trimethylaniline 1.935 g (0.005 mole)
- chlorosulphonic acid 10 ml.
- methylene chloride 30 ml.
- Octane 15 ml.
- Isopropanol 10 ml.
It is added N–methyl–N–benzensulphonyl–2,4,6–trimethylaniline to chlorosulphonic acid at 0 °C was dissolved with stirring and allowed to stand for two days at a temperature close to 0 degree in a tightly closed flask.
After two days it is added in the flask 20 ml of methylene chloride for extraction sulphochloride and dropwised the reaction mass on ice. When the ice melt, the biphasic emulsion is divided into a separatory funnel, the lower layer is collected and washed twice with distilled water from the acid residues. The organic phase is poured into a conical flask and dried with calcium chloride. The organic layer is filtered to remove the calcium chloride and the solvent is evaporated under low heat in a water jet vacuum. The product is triturated with isopropanol, filtered and washed twice with 5 ml of isopropanol.
The sediment from filter is placed in glass and dissolved in methylene chloride. Then it is gently poured 15 ml of octane, trying not to mix solvents. Since methylene chloride is extremely volatile (boiling point — 42 °C) and sulfonyl chlorides and sulfonic acid anilides are practically insoluble in saturated hydrocarbons, interfacial sulfonyl chloride crystals appear. This achieves a high purity of the compound.
Yield 1.18 g (0.00316 mole) of — 63%.
2.4 The cleaning of sulphochlorides of anilides of sulphoacids
These substrates 3–[N(XАrSO2)–NMe]–2,4,6–Me3–C6H2SO2Cl are enough clean for kinetic measurements, but they are additionally recrystallized from dioxane. The sample weighing about 0.5 g is dissolved under heating in 1 ml of hot dioxane and filtered. After cooling the filtrate, the crystallized product is washed with 5 ml isopropanol and dried. The yield was 50–70%.
2.5 Determining the structure of the synthesized compounds
Structure of sulhochlorides of anilides of sulphoacids is confirmed by several ways:
- The definition of molar mass of sulphochloride by acid–base titration;
- The elemental analysis [7] on the presence of active chlorine;
- NMR spectrum of substrates on all studies.
3 The kinetic study the of neutral hydrolysis of 3–[N–(XАrSO2)–NMe]–2,4,6–Me3–C6H2SO2Cl
The kinetics study of the neutral hydrolysis of compounds of the general formula 3–[N(XАrSO2)–NMe]–2,4,6–Me3–C6H2SO2Cl (Х=4–CH3, H, 4–Cl, 3–NO2) 70% (volume) aqueous dioxane and the temperature interval 303–323 K. The effective rate constants and the calculated activation parameters of the transition state are shown in Table. 1.
The reaction of the neutral hydrolysis of investigated substrates is corresponding to equations:
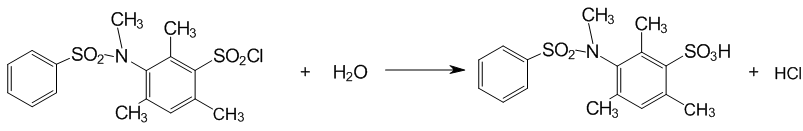
Figure 5 — The hydrolysis of sulphochlorides of anilides of sulphoacids
Table 1 — The effective rate constants keff×104(с-1) and activation parameters for hydrolysis in 70% a.d.
| X | keff×104(с-1) | ΔH≠, kJ/mol |
ΔH≠, J/(mole×К) |
ΔG313≠, kJ/mol |
||
| 303 | 323 | 333 | ||||
| H | 2.5 | 4.68 | 9.59 | 52.0±2.5 | 172±10 | 106±6 |
| 4–Me | 2.53 | 5.49 | 10.9 | 56.9±3.0 | 155±8 | 105±5 |
| 4–Cl | 2.4 | 5.02 | 11.2 | 60.0±1.5 | 139±10 | 104±5 |
| 3–NO2 | 2.24 | 5.04 | 11.3 | 63.0±3.8 | 129±11 | 103±7 |
There is a tendency of increasing reaction rate with increasing electron-drawing nature of the substituent X for all of substrates. It is means that the low electron density at the center of nucleophilic attack — Sulphoatom of Sulphogroup is promoted to process (Table 1).
Substituents X removed from the reaction center and the electronic and steric properties are effected on reaction rate significantly. With increasing the temperature from 303 to 323 K, the difference in reactivity has become more considerable (Table 1). For example, the unsubstituted derivative the ratios k313/k303 и k323/k313 are respectively 1,872 and 2,231 correspondently. In general, the character of the influence of substituent X corresponds to the general regularities of the mechanism of bimolecular nucleophilic substitution SN2-type that previously postulated by many authors [8,9,10] for tetra-coordinated Sulphur atom in derivatives of arenesulphocids. There is the convergence the values of the effective rate constants for all substrates at 303 K. The character of the influence of substituent X changes in the investigated temperature interval. The substituent varying does not significantly effect on value of activation enthalpy ΔH≠, but has considerable effect on entropy factor ΔS≠.
At the same time the increasing of reaction ability of substrates is not explain by a gain in the thermodynamic enthalpy of transition state, it is observed a slight growth of this parameter. The absolute value of the activation enthalpy decreases with increasing electron-drawing character of X.
The fact of the constants of the Gibbs energy values of transition state on 106 kJ/mol level is very interesting.
4. The influence of the structural factors of the substituents on the character of the neutral hydrolysis in 70% a.d.
We have attempted to assess the relationship between the structure of the substrate — the its reactionary by the Hammett equation: [12]:
where ρх — coefficient of the reaction sensitivity to the electronic effect of the substituents X in arenesulphoamide fragment of the molecule;
σ — constants Hammet for characterize the electronic effects of substituents X.
The calculated parameters of the equation are proved the factual absence of correlation with estimated values ρx, which are listed in Table 2.
Table 2 — The calculated parameters by the Hammett equation in 70% a.d. for
3–[N(ХArSO2)–NMe]–2,4,6-Me3–С6НSO2Cl.
| T, К | 3–[N(ХArSO2)–NMe]–2,4,6-Me3–С6НSO2Cl | ArSO2Cl [13] |
| ρx | ρв | |
| 303 | -0.062 | -0.90 |
| 313 | -0.017 | -0.95 |
| 323 | 0.044 | -0.98 |
Comment; Statistical parameters R=0.7÷0.9, S=10-5÷10-2, Δρ=0.04÷0.05
At the same time, the statistical parameters and errors of values ?x indicate the possibility of using parameters of equation (1) for the preliminary analysis. The hydrolysis rate for investigated substrates is comparable with model substrates — the derivatives of benzenesulphochlorides. But the selectivity to X is less for model matters XArSO2Cl (Table 2) [13].
It is probably due to the exclusion of Sulphoatom by bridging group –SO2–N–Me– from the influence X in N–arenesulphonyl fragment and features of the steric structure of the transition and the initial states.
It is observed the clean tendency to convergence of line dependence in Arrhenius axis in the isoparametric region.
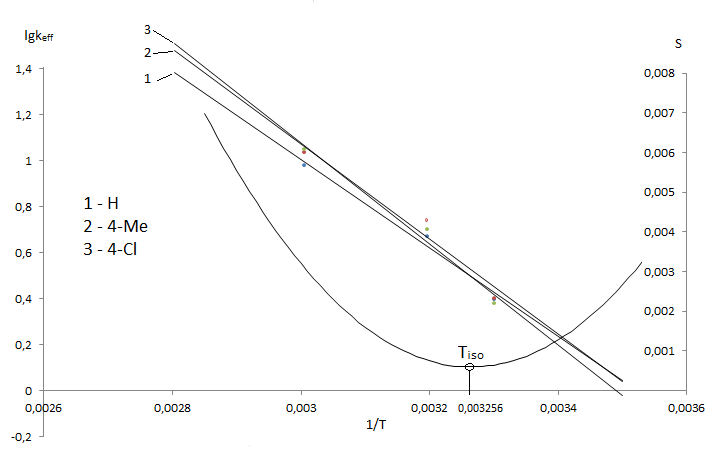
Figure 6 — Arrhenius plot for the hydrolysis in 70% a.d. for 3–[N(ХArSO2)–NMe]–2,4,6-Me3–С6НSO2Cl
The theoretical isokinetic temperature is 306 K.
There is the fairly rare occurrence the isoparametric temperature is in achievable region for the hydrolysis of sulphochlorides of anilides of sulphoacids.
Perhaps, its explain the ambiguity of the kinetic data and activation parameters for transition state.
Thus the interpretation of kinetic specifics should be based on the assessment of the investigated temperature range correspondently to Tiso.
For hydrolysis at T<Tiso is realized the enthalpy control, at T>Tiso — entropy. Of course, the explanation of the structural characteristics of TS should be different at temperatures above and below Tiso. At lower temperatures electronic interactions in TS are prevailed, and at higher — the kinetic peculiarities are determined mainly steric effects in the transition state. The ΔS≠ value is a measure of changes of degree of freedom of internal movement initial state to transition state The value ΔS≠ is become more negative if the system loss the degree of freedom and TS has more difficult configuration [14,15]. Perhaps, the accumulation of methyl groups in sulphochloride molecule part promotes to significant relief of steric interactions at nucleophilic attack.
The packing of TS may be more loose in this cause. And the transition state has not trigonal–bypiramidal structure. It is reflected on increasing of values of free activation energy ΔG for compounds.
5. The development of the technological scheme of the chemical wastewater defenolation
The proposed flowsheet for defenolation department on coke plants is shown in Figure 7.
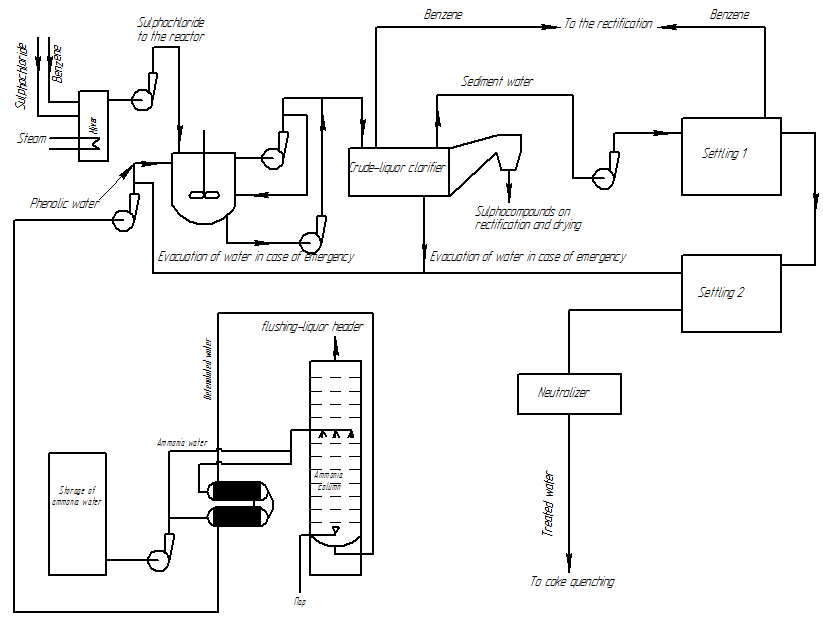
Figure 7 — The flowsheet for department of defenolation on coke plants
Before the defenolation is planned to pre–stripping of volatile ammonia in the ammonia column, which is contained in a water-tar and condensate of primary gas refrigerators of coke enterprises to reduce excess amounts of ammonium salt.
To intensify the process proposed the prior dissolution of benzenesulphonylchloride, heating the mixture to the optimum isokinetic temperature (30 °C), and carrying out the reaction under conditions close to ideal mixing in a capacitive device with mechanical and hydraulic agitation.
The reaction will proceed in colloidal media in well–mixing reactor [14,15]. In the device it is proposed mechanical agitation using a propeller stirrer and hydraulic mixing — multiple pumping fluid through the system reactor — circulator — reactor.
Part of the water removed from the reactor through the drain the pump back to the reactor and another portion is withdrawn description scheme. Since the products of the reaction are insoluble in water but soluble in benzene it is suggested after the reactor take them further crude–liquor clarifier for cleaning of sulphoester precipitates.
Crude-liquor clarifier (Fig. 8) are widely used in the by–product–coking industry for lighten the tar water and tar removal. The fractionation is performed in these devices by gravitational forces. The mixture is fed through a trough 2. The precipitated sulphoester is removed by conveyors 7 via the dumps 6. The treated water output through connection 4. Since the viscosity of benzene is less than one of the resin, the need for heating through the nozzle 8 does not occur.
After crude-liquor clarifier the purified mixture is fed to the two series–connected sump for additional sedimentation. Sump as well as safety container, which can be fed waste water in case of an emergency, or for unloading the machine.
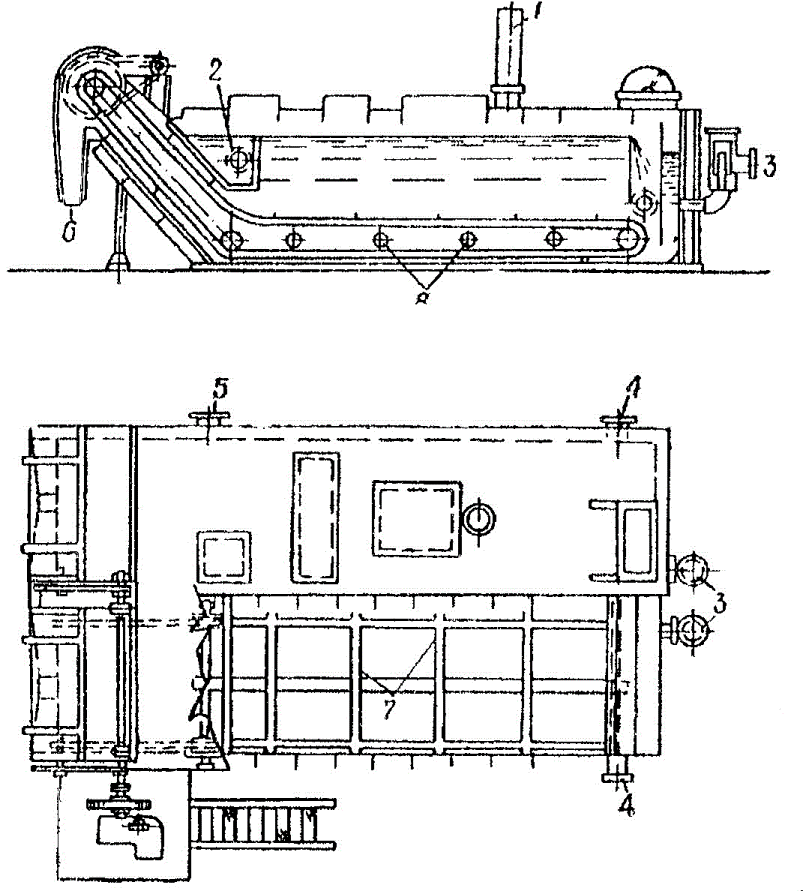
Figure 8 — Crude–liquor clarifier
1 — air tap; 2 — trough; 3 — output of sulphoesters; 4 — output of treated water; 5 — output of mixture; 6 — output of sulphoesters; 7 — scraper conveyor; 8 — steam supply
Pretreated water can be sent to the neutralization for removal the dissolved salt and sulfonic acids, and further for cleaning on wastewater treatment construction, or on the wet quenching of coke. The mixture of sulphoesters and sulfonamides in benzene goes for rectification after settling and benzene is recycled on defenolation and products are amenable to further separation [16].
Conclusion- There are syntesis 12 compounds, which are underscribed in the literature/
- The kinetics of neutral hydrolysis in 70% a.d. in the temperature range 303–333 K was studied.
- The thermodynamic parameters of the transition state for substitution at the sulfonyl center was calculated. The isotemperature region for solvolysis of investigated substrates are found.
- The common SN2 mechanism of substitution SN2 for the study and the model series of substrates was substantiated
- The non–classical transition state in nucleophilic substitution at the sulfonyl center in sulphochlorides of anilides of sulphoacids was proposed.
- The flowsheet of dephenolation department on the basis of the test substrates was presented and substantiated.
References
- Волянская Н.П., Гриценко И.С. Противомикробная активность и фармакологические эффекты фенольньых соединений // Annals of Mechnicov’s Institute, N2, 2005, pp. 3 - 7.
- Харламович Г. Д., Чуркин Ю. В. Фенолы // М.,
Химия
, 1974, 376 с. - Лейбович Р.Е. Технология коксохимического производства: Учебник для техникумов / Лейбович Р.Е., Яковлева Е.И., Филатов А.Б.; Под. ред. Рядинова Н.А. - М.: Металлургия, 1982. – 360 с.
- Boyadzhiev L., Alexandrova S. Dephenolation of Phenol-Containing Waters by Rotating Film Pertraction // Separation Science and Technology, 27(l0), pp 1307-1317.
- Kamenev I., Munter R., Pikkov L. Wastewater treatment in oil shale chemical industry // Oil Shale, 2003, Vol. 20, No. 4, pp. 443-457.
- Giece B., Heuck K. Das reaktivitats-selectivitatprinzip. Anwendung des reaktivitats-selectivitatprinzips auf die sulfonsaureamidbildung // Chem.Ber.-1978.- B.111, №4.- S.1384-1394.
- Мысык Д.Д, Рублева Л.И., Крутько И.Н, Левандовский В.Ю.Влияние структуры N-аренсульфонильного фрагмента на реакционность сульфохлоридов анилидов сульфокислот в условиях нейтрального гидролиза// Вопросы химии и химической технологии.-2004.- № 4.- С.39-42.
- Рублева Л.И. Структура, энергии стерического напряжения и реакционная способность замещенных бензолсульфохлоридов / Л.И. Рублева Н.Н. Максименко С.Н. Лящук Р.В. Визгерт // ЖОрХ.- 1994, т.30, вып.2.-С.261-265.
- Визгерт Р.В., Максименко Н.Н., Рублева Л.И. Особенности реакционной способности стерически затрудненных ароматических сульфохлоридов в реакциях нуклеофильного замещения // Укр.хим.журн.- 1993.-Т.59, № 11.- С.1226-1238.
- Rogne О. Rates of Reaction of Benzenesulphonyl Chloride with Some Nucleophiles in Aqueous Solution // Report. Forsvarets Forskninsinstitut. 1973. N 63. P. 7-30.
- Л. И. Рублева, Д. Д. Мысык, В. Ю. Левандовский, Н. А. Языков // Молодежь в науке - 2009: прил. к журн. «Весці Нацыянальнай акадэміі навук Беларусі». В 5 ч. Ч. 1. Серия химических наук. - Минск: Беларус. навука, 2010. – С. 67 – 71 [Electronic resource]. – Access mode: http://archive.nbuv.gov.ua/..
- Пальм В.А. Основы количесвенной теории органических реакций / Л.: Химия, 1977. – 360с.
- Визгерт Р.В. Реакционная способность пространственно затрудненных ароматических сульфокислот. I. Влияние заместителей на скорость и механизм гидролиза некоторых замещенных бензолсульфохлорида / Р.В.Р.В.Визгерт, Л.И.Р.В.Рублева, Н.Н.Р.В.Максименко // ЖорХ. – 1989.-т.25, вып.4.–С.810–814.
- Мисик Д.Д., Рубльова Л.І., Крутько І.М., Левандовський В.Ю., Язиков М.О. Синтез і реакційна здатність 3–(N–метил–N–аренсульфоніламіно)–4–метоксибензенсульфохлоридів в реакції нейтрального гідролізу // Вопросы химии и хим.технологии.-2008.- №6.- 0,5 у.п.л..
- Rubleva L.I., Levandovsky V.U., Yazikov N.A., Popovich T.S. The application of thermodynamic parameters of transition state for interpretation of mechanism of nucleophilic substitution in sulphochlorides of anilides of sulphoacids // XVIII International Conference on Chemical Thermodynamics in Russia Samara, Russian Federation, October 3–7, 2011 [Electronic resource]. – Access mode: http://ea.donntu.ru..
- Языков Н.А., Рублева Л.И., Левандовский В.Ю. Конструкция и принцип действия реактора для промышленной очистки фенолсодержащих сточных вод // Охорона навколишнього середовища та раціональне використання природних ресурсів. Збірка доповідей VIII Міжнародної конференції аспірантів і студентів.–Т.2– Донецьк: ДонНТУ, ДонНУ, 2009.– С.– 89–90.
