Abstract: "Installation of pyrolytic conversion of solid toalite to produce solid, liquid and gaseous products"
Content
Introduction
Our country is rich in solid fuels, which are an important source of energy and chemical raw materials. The main method of processing is coking with further processing of products. From coal by means of hydrogenation we can get synthetic gasoline, gaseous and liquid hydrocarbons; gasification will produce fuel and raw materials for chemical processing; when semi-coking, synthetic gasoline, gaseous fuels and chemical raw materials[1].
Of all the processes of chemical processing of coal, the process of coking has developed most. This is explained by the role and importance of coking products, mainly coke, which is used in blast furnace, foundry and ferroalloy production. Coke gas and other coking products serve as raw materials for chemical production.
The technology of coke production is constantly developing and improving. Improvement of methods for preparing charge for coking is carried out with the aim of economical use of scarce and expensive highly caking coals and increasing the content of less deficit weakly baked in charge. All coals with high ash content and high sulfur content are enriched [2].
1. Pyrolysis
By the term pyrolysis of coal is commonly understood as a set of processes that occur when the coal is heated, provided that there are no reagents. However, in recent years, under the pyrolysis of coal, the processes occurring with the action of an additional reagent (the so-called hydropyrolysis and oxidative pyrolysis) have also been implied. Often, the term pyrolysis is also used to describe the gasification of coal, although this is not entirely true, since additional reagents are also used. Thermal processing of coal is widely used to produce various carbonaceous solid materials, and liquid and gaseous products. In this regard, depending on the purpose of the final products of pyrolysis, the raw material for processing can be virtually any coal. This is very convenient, since all the extracted coal can be recycled, not a plant for processing solid domestic waste [3].
The processes of pyrolysis of coal were used by mankind since the end of XVIII. At that time, coal was processed to produce materials such as: coal coke used in metallurgy; enriched coals for smokeless burning in furnaces; Light gas used for street lighting. Of course, the technology and process of pyrolysis of coal has not changed since then, but the equipment used for this process, on the contrary, has been improved. Today, as a result of the long evolution of hardware and technical solutions, the process of pyrolysis of coal is characterized by fairly high energy and environmental performance[4].
However, at the same time, one should also take into account the fact that coal pyrolysis products, especially liquid ones, contain in their composition large amounts of organic compounds that contain oxygen, nitrogen and sulfur. For this reason, liquid pyrolysis products of coal can not be used as a synthetic analogue of liquid hydrocarbon fuel without further purification. Therefore, thermal processing of coal is rarely used to produce liquid synthetic fuel as the final product of pyrolysis[5].
It should be mentioned that pyrolysis of coal is carried out at different temperature intervals. The choice of pyrolysis temperature depends on the type of products that need to be obtained eventually. Low-temperature pyrolysis (or semi-coking) is usually performed at 500 - 600 degrees Celsius, and high-temperature pyrolysis (or as it is also called coking) - is produced at 900 - 1100 degrees Celsius. During this process, the following groups of chemical reactions occur:
- Depolymerization of the organic mass of coal to form organic molecules with a lower molecular weight.
- Secondary reactions of transformations of products formed during the pyrolysis process, among them: condensed condensation, polymerization, aromatization, alkylation.
Both groups of chemical reactions proceed both sequentially and in parallel. The final result of the totality of these thermochemical transformations is the formation of liquid gaseous and solid products.[6]
2. Pyrolysis products
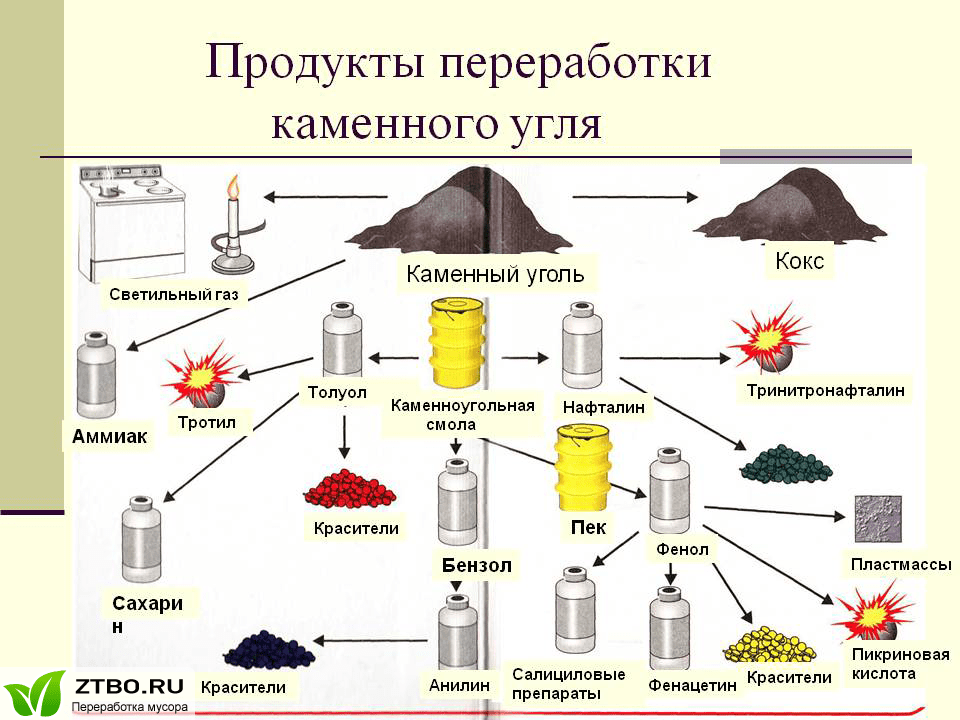
When pyrolysis of coal is hard coke, which is now used mainly in such industries as ferrous and non-ferrous metallurgy. Coke is a more advanced solid fuel than coal, so it is used for the smelting of metals. However, coke, although it is the main product of pyrolysis of coal, is not the most valuable thing that can be extracted from this natural fossil. A by-product of this process is a combined-gas mixture, which contains many chemical compounds. This mixture by condensation is divided into a liquid and a gaseous component, of which, in turn, it is possible to obtain more than 250 chemical compounds. The main liquid product of pyrolysis of coal is coal tar - a black liquid product, which is a complex mixture of organic compounds. From the coal tar by further processing, the following substances are obtained [7].
Picture 1 — Pyrolysis products
2.1 Phenols
Phenols are organic compounds of an aromatic series in whose molecules OH hydroxyl groups are bonded to carbon atoms of an aromatic ring. Most monohydric phenols under normal conditions are colorless crystalline substances with a low melting point and a characteristic odor. Phenols are slightly soluble in water, readily soluble in organic solvents, toxic, when stored in air, they gradually darken as a result of oxidation. Phenol C6H5OH (carbolic acid) - a colorless crystalline substance in the air oxidizes and becomes pink, at ordinary temperature it is boundedly soluble in water, above 66 ° C it mixes with water in any proportions. Phenol - a toxic substance that causes skin burns, is an antiseptic.
2.2 Naphthalene
Naphthalene — C10H8 is a solid crystalline substance with a characteristic odor. It does not dissolve in water, but is readily soluble in benzene, ether, alcohol, chloroform. Physical properties: Density 1.14 g / cm3, melting point 80.26 ° C, boiling point 217.7 ° C, water solubility about 30 mg / l, flash point 79-87 ° C, auto-ignition temperature 525 ° C, molar mass of 128.17052 g / mol. Naphthalene is an important raw material of the chemical industry: it is used for the synthesis of phthalic anhydride, tetralin, decalin, various derivatives of naphthalene. Naphthalene derivatives are used to produce dyes and explosives, in medicine, as insecticide moths in everyday life. Large single crystals are used as scintillators for recording ionizing radiation. Can be used to create synthetic analogues of cannabinoids.
2.3 Anthracene
Anthracene - colorless crystals, melting point 218 ° C. Insoluble in water, soluble in acetonitrile and acetone, it is soluble in benzene upon heating. Chemical properties: It is similar in chemical properties to naphthalene (it is easily nitrated, sulfonated, etc.), but differs from it in that it more easily enters the addition and oxidation reactions. Anthracene can be photodimerized by UV radiation. This leads to a significant change in the properties of the substance. In the dimer, there are two covalent bonds formed as a result of [2 + 2] cycloaddition. The dimer decomposes back into two anthracene molecules upon heating or under UV irradiation with a wavelength below 300 nm. Reversible dimerization and photochromism are the basis for the potential use of mono- and poly-substituted anthracenes. The reaction is sensitive to oxygen. Most other reactions of anthracene also attack the central core, as the most active one.
2.4 Heterocyclic Compounds
Heterocyclic compounds are organic compounds containing rings, which, along with carbon, contain atoms of other elements. Can be considered as carbocyclic compounds with hetero substituents (heteroatoms) in the cycle. The most diverse and well studied aromatic nitrogen-containing heterocyclic compounds. Limit cases of heterocyclic compounds are compounds that do not contain carbon atoms in the cycle, for example, pentazole. Reactivity: The specific features of the reactivity of heterocyclic compounds in comparison with their carbocyclic analogues are due precisely to these hetero substituents. As the heteroatoms, the elements of the second period (N, O) and S are more often, more rarely - Se, P, Si, etc. elements. As in the case of carbocyclic compounds, the most specific properties of heterocyclic compounds are aromatic heterocyclic compounds (heteroaromatic compounds). Unlike carbon atoms of carbocyclic aromatic compounds, heteroatoms can give to the aromatic system not only one (heteroatoms of the pyridine type), but also two (heteroatoms of the pyrrole type) of the electron. Heteroatoms of the pyrrole type are usually included in five-membered rings (pyrrole, furan, thiophene). In one heterocycle, both types of heteroatoms (imidazole, oxazole) can be combined. The specificity of the reactivity of heteroaromatic compounds is determined by the distribution of the electron density in the cycle, which in turn depends on the types of heteroatoms and their electronegativity.
2.5 Technical oils
Technical oils. Problems of environmental safety: When technical oils get into the water, a stable film forms on the surface (as the oils have a density below the water density, insoluble in it and are chemically resistant), which prevents the saturation of water with oxygen (aeration), leading to the death of aquatic inhabitants. Oil ingress on the ground also leads to the drying of plants (for example, the popular way of destroying undesirable trees - watering them with used engine oil), earthworms, other organisms has long been known. Thus, the strong toxic effect of oils on nature combined with their wide application creates an acute problem of utilization and processing of technical oils, as well as more strict compliance with the rules for their operation and transportation. Here it should be noted about the re-processing and use of technical oils, which now exists. First of all, it is necessary to mention the regeneration and secondary use of transformer oils (taking into account that the transformer park is quite large). Spent motor oils are used as a fuel - a number of firms produce special furnaces for this purpose.
2.6 Synthetic fuel
Synthetic fuel - hydrocarbon fuel, which differs from conventional fuel by the production process, that is, obtained by processing the raw material, which before processing has unsuitable characteristics for the consumer. Typically, this term refers to a liquid fuel derived from solid fuel or from gaseous fuel.
However, it should be noted that oil and liquid fuel obtained by pyrolysis of coal are unsuitable for use in internal combustion engines, since they contain many impurities in their composition. For this reason, these pyrolysis products need further purification for further use. And this significantly increases the cost of these pyrolysis products, making their production not very cost-effective. The gaseous pyrolysis product of coal is the so-called pyrolysis gas, which is a mixture of combustible gases and various chemical compounds. In many countries of the world, pyrolysis gas is now used as an alternative source of energy, primarily thermal energy. If for us this technology is quite new, then in some European countries pyrolysis gas has long become a habitual fuel. In addition, pyrolysis gas as well as coal tar can be used to produce various chemical compounds. Thus, benzene, phenol and other substances are released from this gas[8].
3.Installation diagram and principle of operation
The aim of the work is to create a laboratory installation for high-temperature pyrolysis (coking) of coal, which allows obtaining a solid residue, resin and coke oven gas.
To carry out the experiment, a furnace was made (N = 1600 W, t = 1100 ° C), allowing to work with small weights, the basis of which is a reactor tube (ceramic) with a diameter of 20 mm and a length of 600 mm. The tube was wound with a nichrome wire, the turns of which were located at a distance of 2 mm from each other. A 2 mm thick nichrome wire was used to create the desired temperature inside the furnace. The section of nichrome wire on the surface of the tube was covered with a mixture of aluminosilicate and clay for thermal insulation. The reactor-tube was placed in a case with a diameter of 150 mm, which was covered with covers. Free space in the case was also filled with asbestos for thermal insulation. At this stage, the thermocouple was calibrated to ensure that the instrument is accurate. Calibration is carried out at 5-6 points using special instruments (exemplary thermocouple) or the melting point of metals, the melting point of which is less than 1100 ° C (tin, lead, aluminum, zinc, copper).
To carry out the experiment, a ceramic boat with pre-crushed coal is placed in the middle of the furnace. On one side of the furnace a thermocouple is connected, and on the other side the absorber part of the installation. The furnace is connected to the voltage regulator and the coking process is started. The beginning of the reaction is judged by the separation of coke oven gas and the condensation of coal tar in the reservoir for collecting the liquid fraction. The first portions of coke oven gas are passed through a solution of bromine water, which decolorizes, which proves the presence of unsaturated compounds. After the completion of the process, we conduct an analysis of the coking products.
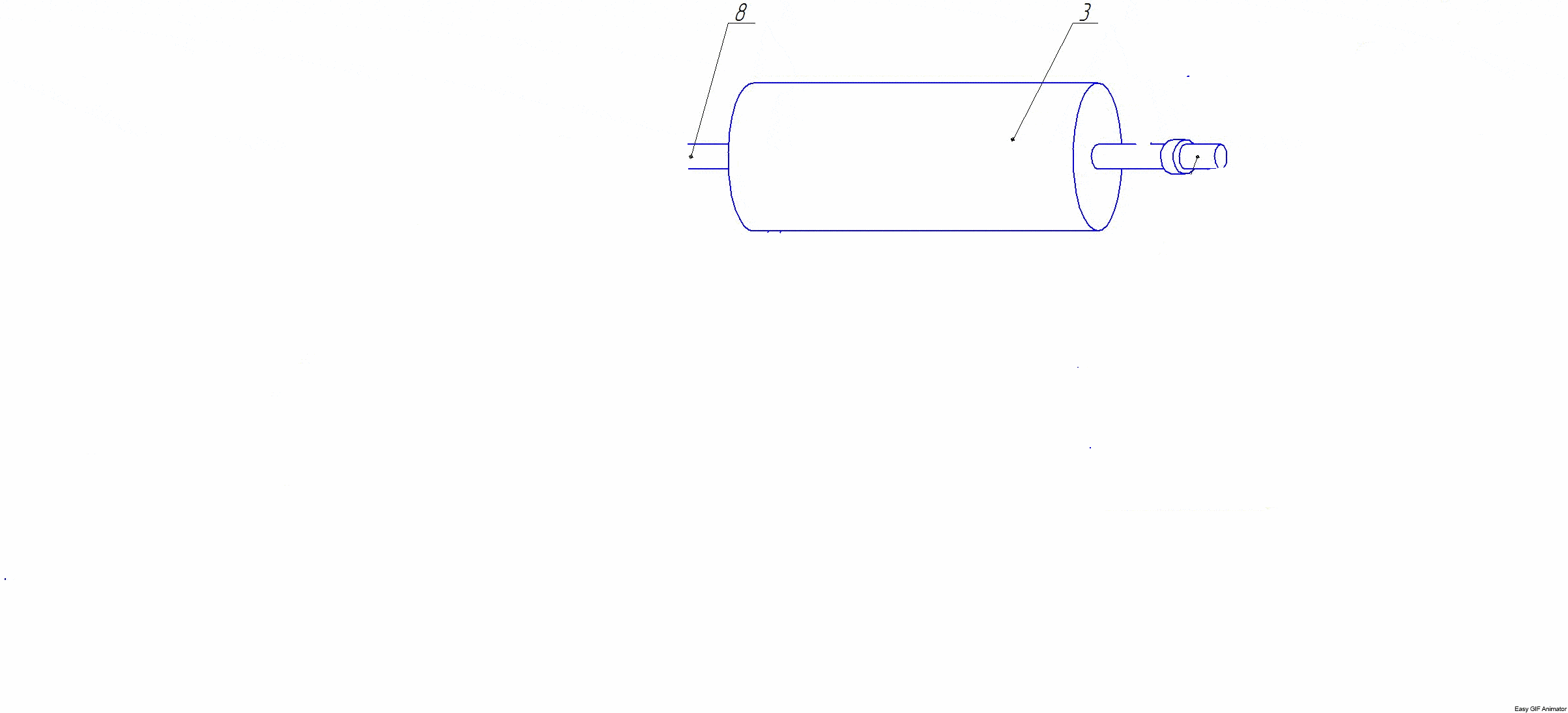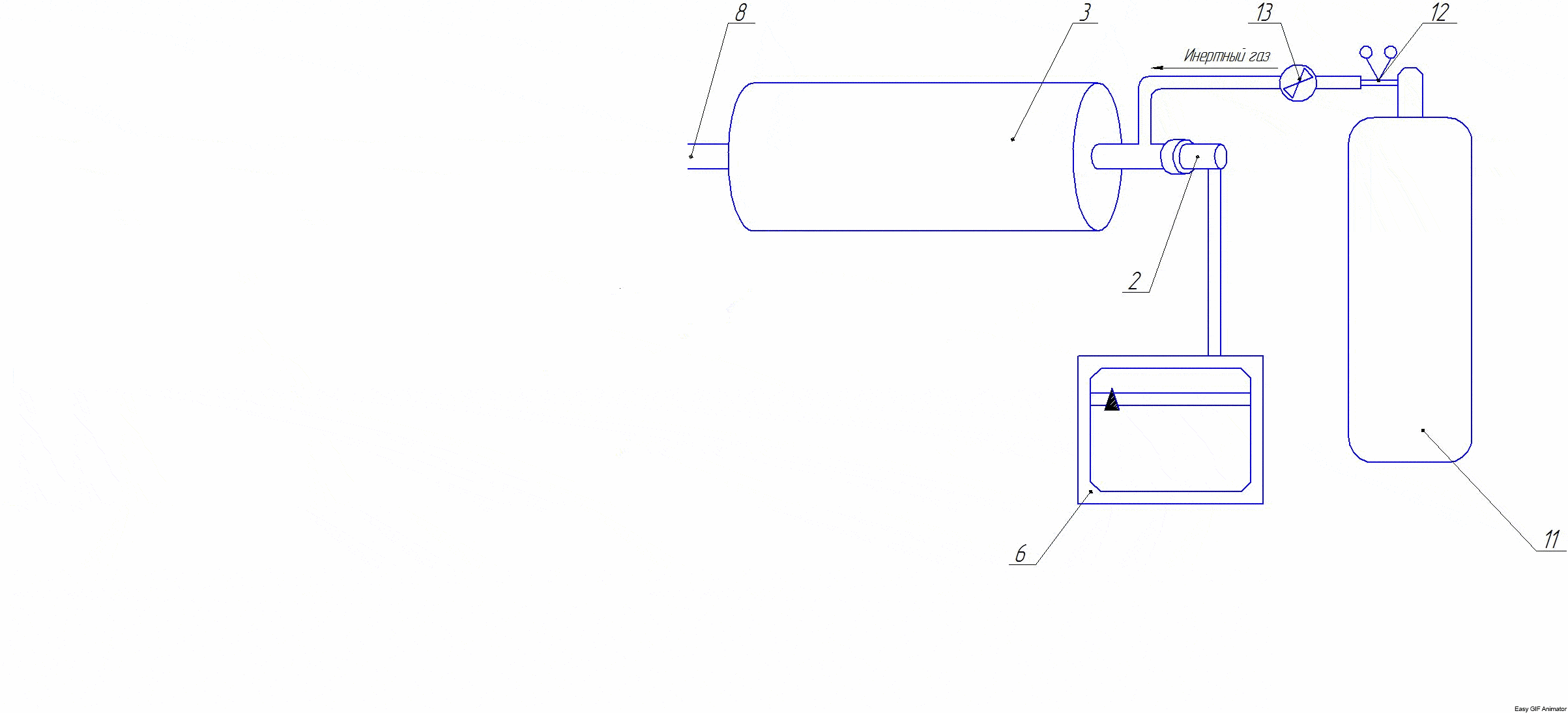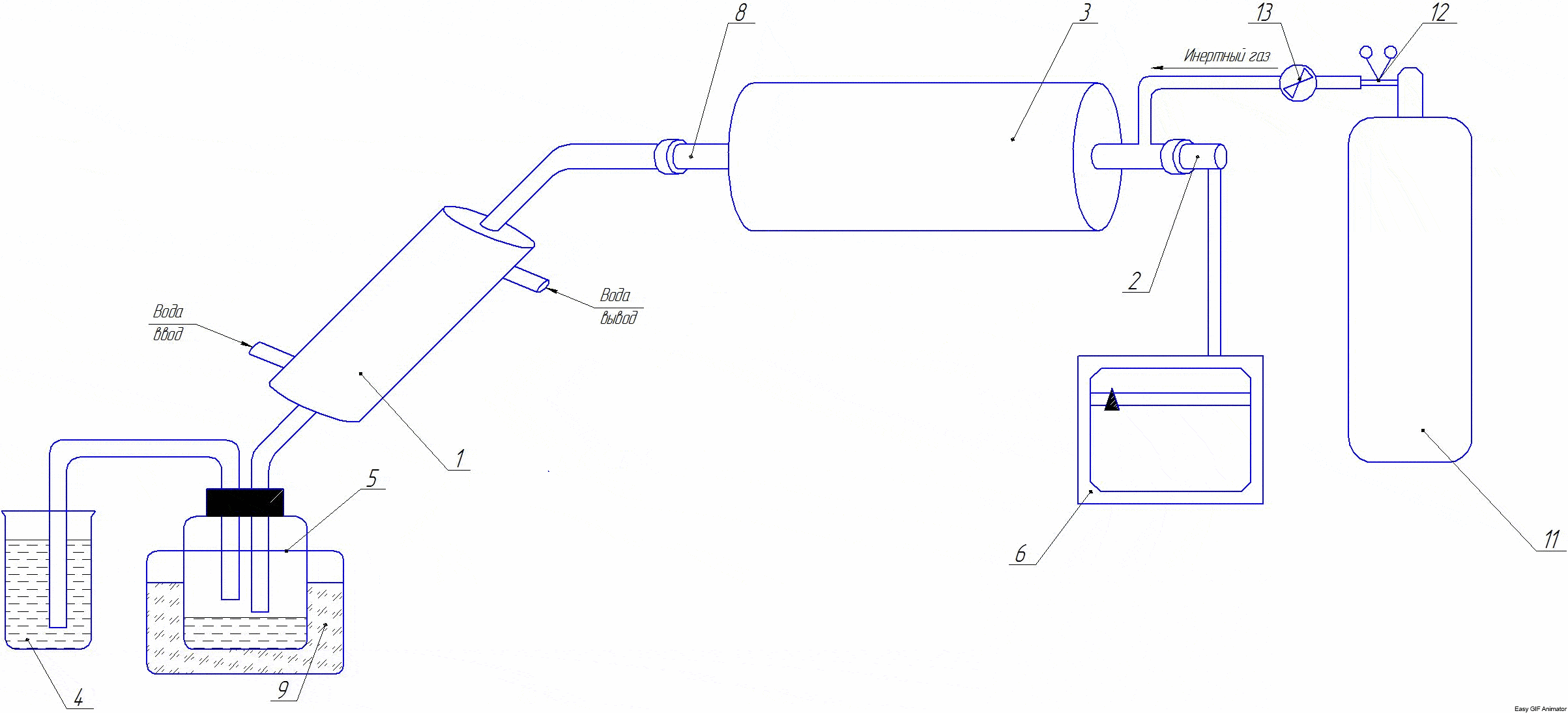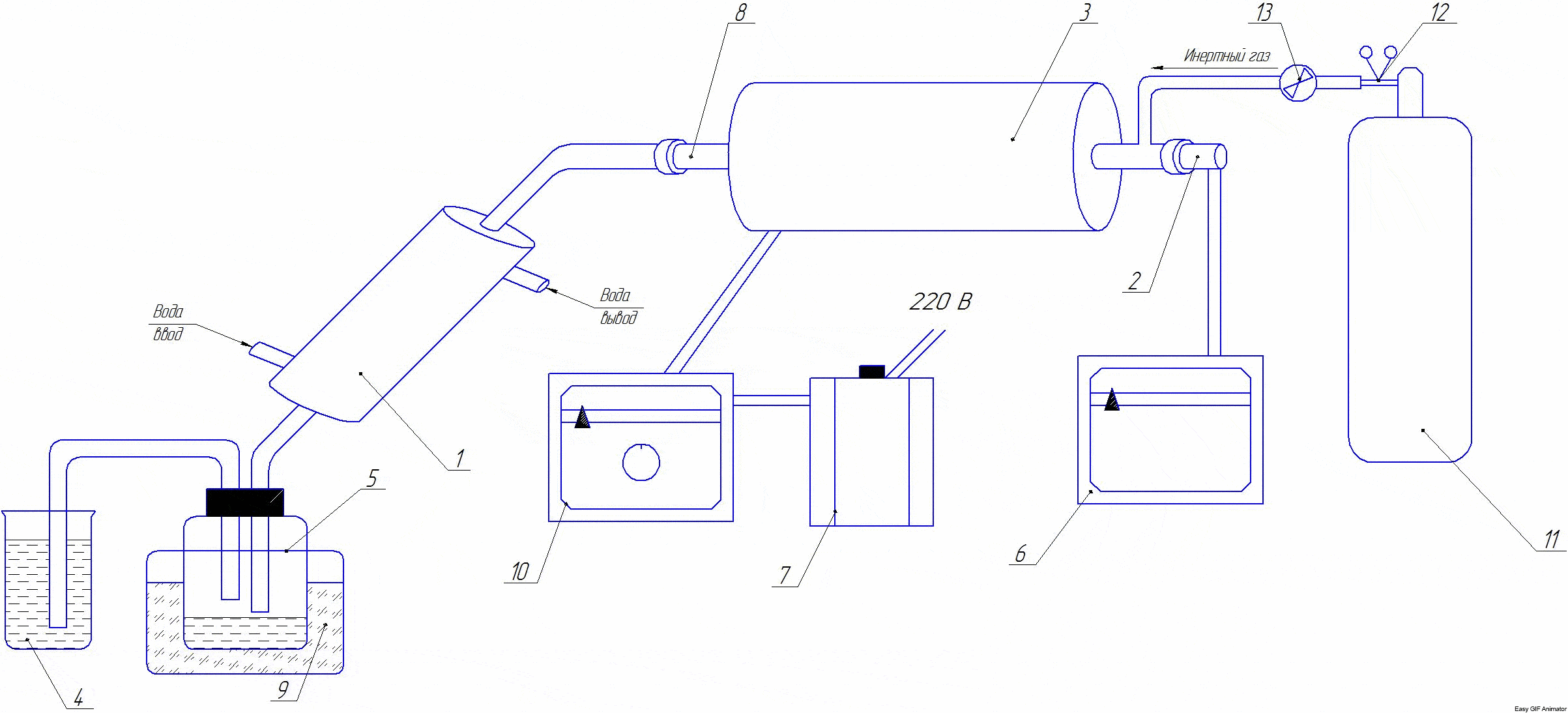
Рicture 2 — Installation diagram
The installation consists of: 1 - refrigerator; 2 - thermocouple; 3 - the furnace; 4 - gas meter; 5 - container for collecting liquid fraction; 6 - the resistor; 7 - the transformer; 8 - reactor-tube; 9 - a container with ice; 10- a balloon with an inert gas; 11- thermotygulator; 12- reducer; 13- catch.
At the beginning of the experiment, the comminuted coals are weighed and loaded into the reactor-tube 8. The tube is closed with a lid. The opposite end of the tube is closed by a stopper with a withdrawn tube for connecting the refrigerator. A container weighed on a technical scale can be connected to the refrigerator to collect liquid fraction 5. If necessary, an inert medium in the reactor can be created with the help of an inert gas placed in the container 10. The tube furnace is heated to the required temperature (the temperature is set with the help of a transformer 7). The temperature in the furnace can also be set and controlled by the thermostat 11. The required process temperature is created by the tubular furnace 3, which is connected to the mains via the autotransformer 7. The temperature in the furnace is controlled by a thermocouple 2 connected to the resistor 6. The pyrolysis time is started after reaching the set temperature. Liquid and gaseous pyrolysis products are discharged through the refrigerator 1 to a capacity of 5.4, respectively.
In the bottom work, the significance of the pyrolysis process, the products obtained during pyrolysis, their physical and chemical properties were considered. The scheme of the laboratory installation of pyrolytic conversion of solid fuel, and a description of its operation are considered.
When writing this essay, the master's work is not yet complete. Final completion: May 2018. The full text of the work and materials on the topic can be obtained from the author after the indicated date.